Metabolic heterogeneity confers differences in melanoma metastatic potential
- PMID: 31853067
- PMCID: PMC6930341
- DOI: 10.1038/s41586-019-1847-2
Metabolic heterogeneity confers differences in melanoma metastatic potential
Abstract
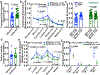
Metastasis requires cancer cells to undergo metabolic changes that are poorly understood1-3. Here we show that metabolic differences among melanoma cells confer differences in metastatic potential as a result of differences in the function of the MCT1 transporter. In vivo isotope tracing analysis in patient-derived xenografts revealed differences in nutrient handling between efficiently and inefficiently metastasizing melanomas, with circulating lactate being a more prominent source of tumour lactate in efficient metastasizers. Efficient metastasizers had higher levels of MCT1, and inhibition of MCT1 reduced lactate uptake. MCT1 inhibition had little effect on the growth of primary subcutaneous tumours, but resulted in depletion of circulating melanoma cells and reduced the metastatic disease burden in patient-derived xenografts and in mouse melanomas. In addition, inhibition of MCT1 suppressed the oxidative pentose phosphate pathway and increased levels of reactive oxygen species. Antioxidants blocked the effects of MCT1 inhibition on metastasis. MCT1high and MCT1-/low cells from the same melanomas had similar capacities to form subcutaneous tumours, but MCT1high cells formed more metastases after intravenous injection. Metabolic differences among cancer cells thus confer differences in metastatic potential as metastasizing cells depend on MCT1 to manage oxidative stress.
Figures






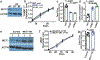






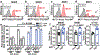
Comment in
-
Surviving stress during metastasis.Nat Rev Cancer. 2020 Mar;20(3):139. doi: 10.1038/s41568-020-0243-4. Nat Rev Cancer. 2020. PMID: 32020065 No abstract available.
References
-
- Le Gal K et al. Antioxidants can increase melanoma metastasis in mice. Sci Transl Med 7, 308re308 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

