Cyclooxygenase-2 in Endometriosis
- PMID: 31853218
- PMCID: PMC6909960
- DOI: 10.7150/ijbs.35128
Cyclooxygenase-2 in Endometriosis
Abstract
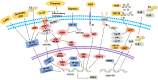
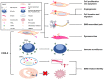
Endometriosis (EMS) is the most common gynecological disease in women of reproductive age, and it is associated with chronic pelvic pain, dyspareunia and infertility. As a consequence of genetic, immune and environmental factors, endometriotic lesions have high cyclooxygenase (COX)-2 and COX-2-derived prostaglandin E2 (PGE2) biosynthesis compared with the normal endometrium. The transcription of the PTGS2 gene for COX-2 is associated with multiple intracellular signals, which converge to cause the activation of mitogen-activated protein kinases (MAPKs). COX-2 expression can be regulated by several factors, such as estrogen, hypoxia, proinflammatory cytokines, environmental pollutants, metabolites and metabolic enzymes, and platelets. High concentrations of COX-2 lead to high cell proliferation, a low level of apoptosis, high invasion, angiogenesis, EMS-related pain and infertility. COX-2-derived PGE2 performs a crucial function in EMS development by binding to EP2 and EP4 receptors. These basic findings have contributed to COX-2-targeted treatment in EMS, including COX-2 inhibitors, hormone drugs and glycyrrhizin. In this review, we summarize the most recent basic research in detail and provide a short summary of COX-2-targeted treatment.
Keywords: COX-2; PGE2; endometriosis; estrogen; pain.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Frackiewicz EJ. Endometriosis: an overview of the disease and its treatment. J Am Pharm Assoc (Wash) 2000;40(5):645–57. quiz 99-702. - PubMed
-
- Goumenou AG, Matalliotakis IM, Tzardi M, Fragouli YG, Mahutte NG, Arici A. Apoptosis and differential expression of apoptosis-related proteins in endometriotic glandular and stromal cells. J Soc Gynecol Investig. 2004;11(5):318–22. - PubMed
-
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24(2):235–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

