Learned sensing: jointly optimized microscope hardware for accurate image classification
- PMID: 31853404
- PMCID: PMC6913384
- DOI: 10.1364/BOE.10.006351
Learned sensing: jointly optimized microscope hardware for accurate image classification
Abstract
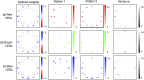
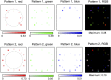
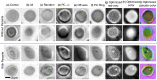
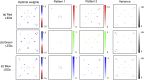
Since its invention, the microscope has been optimized for interpretation by a human observer. With the recent development of deep learning algorithms for automated image analysis, there is now a clear need to re-design the microscope's hardware for specific interpretation tasks. To increase the speed and accuracy of automated image classification, this work presents a method to co-optimize how a sample is illuminated in a microscope, along with a pipeline to automatically classify the resulting image, using a deep neural network. By adding a "physical layer" to a deep classification network, we are able to jointly optimize for specific illumination patterns that highlight the most important sample features for the particular learning task at hand, which may not be obvious under standard illumination. We demonstrate how our learned sensing approach for illumination design can automatically identify malaria-infected cells with up to 5-10% greater accuracy than standard and alternative microscope lighting designs. We show that this joint hardware-software design procedure generalizes to offer accurate diagnoses for two different blood smear types, and experimentally show how our new procedure can translate across different experimental setups while maintaining high accuracy.
© 2019 Optical Society of America under the terms of the OSA Open Access Publishing Agreement.
Conflict of interest statement
The authors declare that there are no conflicts of interest related to this article.
Figures




References
-
- Buggenthin F., Buettner F., Hoppe P. S., Endele M., Kroiss M., Strasser M., Schwarzfischer M., Loeffler D., Kokkaliaris K. D., Hilsenbeck O., Schroeder T., Theis F. J., Marr C., “Prospective identification of hematopoietic lineage choice by deep learning,” Nat. Methods 14(4), 403–406 (2017). 10.1038/nmeth.4182 - DOI - PMC - PubMed
-
- Apthorpe N., Riordan A., Aguilar R., Homann J., Gu Y., Tank D., Seung H. S., “Automatic neuron detection in calcium imaging data using convolutional networks,” in Advances in Neural Information Processing Systems 29, Lee D. D., Sugiyama M., Luxburg U. V., Guyon I., Garnett R., eds. (Curran Associates, Inc., 2016), pp. 3270–3278.
LinkOut - more resources
Full Text Sources
