Surfactant-Stripped Pheophytin Micelles for Multimodal Tumor Imaging and Photodynamic Therapy
- PMID: 31853516
- PMCID: PMC6919654
- DOI: 10.1021/acsabm.8b00703
Surfactant-Stripped Pheophytin Micelles for Multimodal Tumor Imaging and Photodynamic Therapy
Abstract
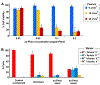
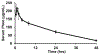
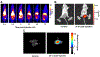
Porphyrin-based nanomaterials can inherently integrate multiple contrast imaging functionalities with phototherapeutic capabilities. We dispersed pheophytin (Pheo) into Pluronic F127 and carried out low-temperature surfactant-stripping to remove the bulk surfactant. Surfactant-stripped Pheo (ss-Pheo) micelles exhibited a similar size, but higher near-infrared fluorescence, compared to two other nanomaterials also with high porphyrin density (surfactant-stripped chlorophyll micelles and porphysomes). Singlet oxygen generation, which was higher for ss-Pheo, enabled photodynamic therapy (PDT). ss-Pheo provided contrast for photoacoustic and fluorescence imaging, and following seamless labeling with 64Cu, was used for positron emission tomography. ss-Pheo had a long blood circulation and favorable accumulation in an orthotopic murine mammary tumor model. Trimodal tumor imaging was demonstrated, and PDT resulted in delayed tumor growth.
Keywords: fluorescence; micelles; pheophytin; photoacoustic; photodynamic therapy; positron emission tomography.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
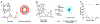
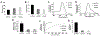
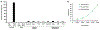
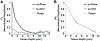
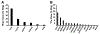
Similar articles
-
Surfactant-Stripped Frozen Pheophytin Micelles for Multimodal Gut Imaging.Adv Mater. 2016 Oct;28(38):8524-8530. doi: 10.1002/adma.201602373. Epub 2016 Jul 11. Adv Mater. 2016. PMID: 27396479 Free PMC article.
-
Surfactant-Stripped Micelles of Near Infrared Dye and Paclitaxel for Photoacoustic Imaging Guided Photothermal-Chemotherapy.Small. 2018 Nov;14(44):e1802991. doi: 10.1002/smll.201802991. Epub 2018 Oct 4. Small. 2018. PMID: 30286285
-
Surfactant-Stripped Micelles for NIR-II Photoacoustic Imaging through 12 cm of Breast Tissue and Whole Human Breasts.Adv Mater. 2019 Oct;31(40):e1902279. doi: 10.1002/adma.201902279. Epub 2019 Aug 15. Adv Mater. 2019. PMID: 31414515 Free PMC article.
-
Surfactant-stripped naphthalocyanines for multimodal tumor theranostics with upconversion guidance cream.Nanoscale. 2017 Mar 9;9(10):3391-3398. doi: 10.1039/c6nr09321c. Nanoscale. 2017. PMID: 28247896 Free PMC article.
-
Porphyrin-Based Nanomedicines for Cancer Treatment.Bioconjug Chem. 2019 Jun 19;30(6):1585-1603. doi: 10.1021/acs.bioconjchem.9b00231. Epub 2019 May 7. Bioconjug Chem. 2019. PMID: 31023011 Review.
Cited by
-
A Review of the Efficacy of Nanomaterial-Based Natural Photosensitizers to Overcome Multidrug Resistance in Cancer.Pharmaceutics. 2024 Aug 24;16(9):1120. doi: 10.3390/pharmaceutics16091120. Pharmaceutics. 2024. PMID: 39339158 Free PMC article. Review.
-
Enhancement of Inhibitory Activity by Combining Allosteric Inhibitors Putatively Binding to Different Allosteric Sites on Cathepsin K.Molecules. 2023 May 19;28(10):4197. doi: 10.3390/molecules28104197. Molecules. 2023. PMID: 37241936 Free PMC article.
-
A self-assembled nanophotosensitizer targets lysosomes and induces lysosomal membrane permeabilization to enhance photodynamic therapy.Chem Sci. 2023 Apr 19;14(19):5106-5115. doi: 10.1039/d3sc00455d. eCollection 2023 May 17. Chem Sci. 2023. PMID: 37206384 Free PMC article.
References
-
- Lovell JF; Liu TWB; Chen J; Zheng G Activatable Photosensitizers for Imaging and Therapy. Chem. Rev. 2010, 110 (5), 2839–2857. - PubMed
-
- Kessel D Photodynamic Therapy: From the Beginning. Photodiagn. Photodyn. Ther. 2004, 1 (1), 3–7. - PubMed
-
- Robertson CA; Evans DH; Abrahamse H Photodynamic Therapy (PDT): A Short Review on Cellular Mechanisms and Cancer Research Applications for PDT. J. Photochem. Photobiol., B 2009, 96 , 1–8. - PubMed
-
- Berg K; Selbo PK; Weyergang A; Dietze A; Prasmickaite L; Bonsted A; Engesaeter B0; Angell-Petersen E; Warloe T; Frandsen N; Høgset A Porphyrin-Related Photosensitizers for Cancer Imaging and Therapeutic Applications. J. Microsc. 2005, 218 , 133–147. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
