Assessment of hepatocellular carcinoma treatment response with LI-RADS: a pictorial review
- PMID: 31853668
- PMCID: PMC6920285
- DOI: 10.1186/s13244-019-0801-z
Assessment of hepatocellular carcinoma treatment response with LI-RADS: a pictorial review
Abstract
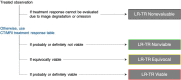
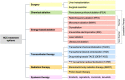
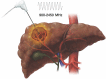
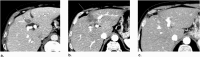
Computed tomography (CT) and magnetic resonance imaging (MRI) play critical roles for assessing treatment response of hepatocellular carcinoma (HCC) after locoregional therapy. Interpretation is challenging because posttreatment imaging findings depend on the type of treatment, magnitude of treatment response, time interval after treatment, and other factors. To help radiologists interpret and report treatment response in a clear, simple, and standardized manner, the Liver Imaging Reporting and Data System (LI-RADS) has developed a Treatment Response (LR-TR) algorithm. Introduced in 2017, the system provides criteria to categorize response of HCC to locoregional treatment (e.g., chemical ablation, energy-based ablation, transcatheter therapy, and radiation therapy). LR-TR categories include Nonevaluable, Nonviable, Equivocal, and Viable. LR-TR does not apply to patients on systemic therapies. This article reviews the LR-TR algorithm; discusses locoregional therapies for HCC, treatment concepts, and expected posttreatment findings; and illustrates LI-RADS treatment response assessment with CT and MRI.
Keywords: Computed tomography; Hepatocellular carcinoma; LI-RADS Treatment Response; LIRADS; Locoregional; Magnetic resonance imaging.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures










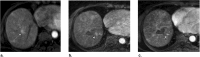

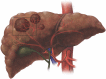

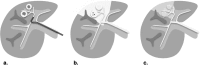



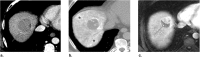
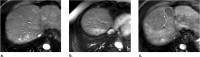

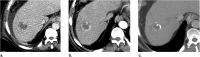


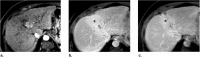




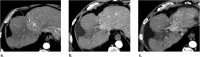
References
-
- Kielar Ania, Fowler Kathryn J., Lewis Sara, Yaghmai Vahid, Miller Frank H., Yarmohammadi Hooman, Kim Charles, Chernyak Victoria, Yokoo Takeshi, Meyer Jeffrey, Newton Isabel, Do Richard K. Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm. Abdominal Radiology. 2017;43(1):218–230. doi: 10.1007/s00261-017-1281-6. - DOI - PMC - PubMed
-
- American College of Radiology (2018) Liver Imaging Reporting and Data System version 2018 Manual. Available via: https://www.acr.org/-/media/ACR/Files/Clinical-Resources/LIRADS/LI-RADS-.... Accessed 11th June 2019.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

