Life in the lumen: The multivesicular endosome
- PMID: 31854087
- PMCID: PMC7004041
- DOI: 10.1111/tra.12715
Life in the lumen: The multivesicular endosome
Abstract
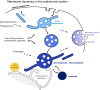
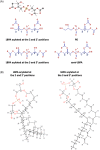
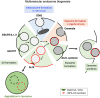
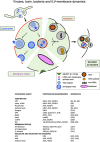
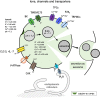
The late endosomes/endo-lysosomes of vertebrates contain an atypical phospholipid, lysobisphosphatidic acid (LBPA) (also termed bis[monoacylglycero]phosphate [BMP]), which is not detected elsewhere in the cell. LBPA is abundant in the membrane system present in the lumen of this compartment, including intralumenal vesicles (ILVs). In this review, the current knowledge on LBPA and LBPA-containing membranes will be summarized, and their role in the control of endosomal cholesterol will be outlined. Some speculations will also be made on how this system may be overwhelmed in the cholesterol storage disorder Niemann-Pick C. Then, the roles of intralumenal membranes in endo-lysosomal dynamics and functions will be discussed in broader terms. Likewise, the mechanisms that drive the biogenesis of intralumenal membranes, including ESCRTs, will also be discussed, as well as their diverse composition and fate, including degradation in lysosomes and secretion as exosomes. This review will also discuss how intralumenal membranes are hijacked by pathogenic agents during intoxication and infection, and what is the biochemical composition and function of the intra-endosomal lumenal milieu. Finally, this review will allude to the size limitations imposed on intralumenal vesicle functions and speculate on the possible role of LBPA as calcium chelator in the acidic calcium stores of endo-lysosomes.
Keywords: ALIX; ESCRTs; Niemann-pick C; anthrax; bis(monoacylglycero)phosphate BMP; calcium store; cholesterol; enveloped virus; exosome; intralumenal vesicle ILV; lipidomics; lysobisphosphatidic acid; lysosome; lysosome storage disease; multivesicular endosome; pathogen; penetration; toxin.
© 2019 The Authors. Traffic published by John Wiley & Sons Ltd.
Figures
References
-
- Scott CC, Vacca F, Gruenberg J. Endosome maturation, transport and functions. Semin Cell Dev Biol. 2014;31:2‐10. - PubMed
-
- Simonetti B, Cullen PJ. Endosomal sorting: architecture of the Retromer coat. Curr Biol. 2018;28(23):R1350‐R1352. - PubMed
-
- Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol. 2012;28:337‐362. - PubMed
-
- Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8(8):622‐632. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

