Doppler US and CT Diagnosis of Nutcracker Syndrome
- PMID: 31854150
- PMCID: PMC6923211
- DOI: 10.3348/kjr.2019.0084
Doppler US and CT Diagnosis of Nutcracker Syndrome
Abstract
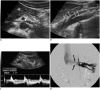
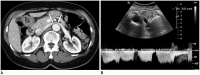
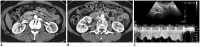
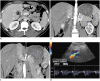
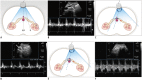
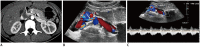
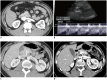
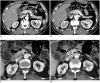
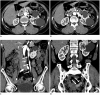
Nutcracker syndrome (NCS) is a syndrome caused by compression of the left renal vein (LRV), between the abdominal aorta and the superior mesenteric artery, resulting in hypertension of the LRV and hematuria. Doppler ultrasonography (US) has been commonly used for the diagnosis of NCS. However, several technical issues, such as Doppler angle and sample volume, need to be considered to obtain satisfactory results. In addition, morphologic changes of the LRV and a jetting phenomenon across the aortomesenteric portion of the LRV on contrast-enhanced computed tomography (CECT) are diagnostic clues of NCS. With proper Doppler US and CECT, NCS can be diagnosed noninvasively.
Keywords: Contrast-enhanced CT; Doppler US; Left renal vein; Nutcracker syndrome; Renal vein hypertension.
Copyright © 2019 The Korean Society of Radiology.
Conflict of interest statement
The author has no potential conflicts of interest to disclose.
Figures
References
-
- El-Sadr AR, Mina E. Anatomical and surgical aspects in the operative management of varicocele. Urol Cutaneous Rev. 1950;54:257–262. - PubMed
-
- Chait A, Matasar KW, Fabian CE, Mellins HZ. Vascular impressions on the ureters. Am J Roentgenol Radium Ther Nucl Med. 1971;111:729–749. - PubMed
-
- de Schepper A. [“Nutcracker” phenomenon of the renal vein and venous pathology of the left kidney] J Belge Radiol. 1972;55:507–511. - PubMed
-
- Welsch T, Büchler MW, Kienle P. Recalling superior mesenteric artery syndrome. Dig Surg. 2007;24:149–156. - PubMed
-
- Ananthan K, Onida S, Davies AH. Nutcracker syndrome: an update on current diagnostic criteria and management guidelines. Eur J Vasc Endovasc Surg. 2017;53:886–894. - PubMed

