Hierarchically structured hydrogels utilizing multifunctional assembling peptides for 3D cell culture
- PMID: 31854388
- PMCID: PMC7439559
- DOI: 10.1039/c9bm01894h
Hierarchically structured hydrogels utilizing multifunctional assembling peptides for 3D cell culture
Abstract
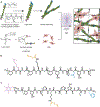
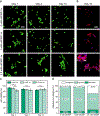
Approaches for the creation of soft materials, particularly hydrogels, with hierarchical structure are of interest in a variety of applications owing to their unique properties. In the context of tissue mimics, hydrogels with multiscale structures more accurately capture the complexities of tissues within the body (e.g., fibrous collagen-rich microenvironments). However, cytocompatible fabrication of such materials with hierarchical structures and independent control of mechanical and biochemical properties remains challenging and is needed for probing and directing cell-microenvironment interactions for three-dimensional (3D) cell encapsulation and culture applications. To address this, we have designed innovative multifunctional assembling peptides: these unique peptides contain a core block that mimics the structure of collagen for achieving relevant melting temperatures; 'sticky' ends to promote assembly of long fibrils; and a biocompatible reactive handle that is orthogonal to assembly to allow the formation of desired multiscale structures and their subsequent rapid, light-triggered integration within covalently crosslinked synthetic hydrogels. Nano- to micro-fibrils were observed to form in physiologically-relevant aqueous solutions, where both underlying peptide chemical structure and assembly conditions were observed to impact the resulting fibril sizes. These assembled structures were 'locked' into place and integrated as linkers within cell-degradable, bioactive hydrogels formed with photoinitiated thiol-ene 'click' chemistry. Hydrogel compositions were identified for achieving robust mechanical properties like those of soft tissues while also retaining higher ordered structures after photopolymerization. The utility of these innovative materials for 3D cell culture was demonstrated with human mesenchymal stem cells, where cell morphologies reminiscent of responses to assembled native collagen were observed now with a fully synthetic material. Using a bottom-up approach, a new materials platform has been established that combines the advantageous properties of covalent and assembling chemistries for the creation of synthetic hydrogels with controllable nanostructure, mechanical properties, and biochemical content.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

