Aminoglycosides: Time for the Resurrection of a Neglected Class of Antibacterials?
- PMID: 31855407
- PMCID: PMC7024022
- DOI: 10.1021/acsinfecdis.9b00441
Aminoglycosides: Time for the Resurrection of a Neglected Class of Antibacterials?
Abstract
This Viewpoint addresses the question of whether, after years of declining use, the time is ripe for renewed investigations into the aminoglycoside class of antibiotics for the development of novel therapeutic agents for the treatment of drug-resistant bacterial infections, particularly of the Gram-negative type. The reasons underlying the decline in use of the aminoglycosides are briefly considered and found to be outweighed by the ever-increasing clinical need for improved antibacterials with which to combat modern day multidrug resistant pathogens. The potential of the aminoglycosides builds on their well-established pharmacokinetics/pharmacodynamics (PK/PD), mechanisms of action, toxicity, and resistance and the extensive existing structure-activity relationship (SAR) databases, which permit rational, informed drug design. When coupled with the power of modern synthetic organic chemistry and improved funding scenarios, these multiple attributes open the door for the development of structurally novel, potent, and less toxic aminoglycosides to address the pressing societal problem of multidrug-resistant (MDR) infectious disease.
Conflict of interest statement
Transparency declaration
The authors are co-founders of and have an equity interest in Juvabis AG, a biotech start-up operating in the area of aminoglycoside antibiotics.
Figures
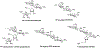
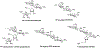
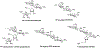
References
-
- Kumarasamy KK; Toleman MA; Walsh TR; Bagaria J; Butt F; Balakrishnan R; Chaudhary U; Doumith M; Giske CG; Irfan S; Krishnan P; Kumar AV; Maharjan S; Mushtaq S; Noorie T; Paterson DL; Pearson A; Perry C; Pike R; Rao B; Ray U; Sarma JB; Sharma M; Sheridan E; Thirunarayan MA; Turton J; Upadhyay S; Warner M; Welfare W; Livermore DM; Woodford N, Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect. Dis 2010, 10, 597–602. - PMC - PubMed
-
- ECDC European Centre for Disease Prevention and Control. Regional Outbreak of New Delhi Metallo-beta-lactamase Producing Carbapenen-resistant Enterobacteriaceae, Italy, 2018–2019; ECDC: Stockholm, 2019.
-
- Davies J; Gorini L; Davies BD, Misreading of RNA Codewords Induced by Aminoglycoside Antibiotics. Mol. Pharmacol 1965, 1, 93–106. - PubMed
-
- Cabañas MJ; Vázquez D; Modolell J, Inhibition of Ribosomal Translocation by Aminoglycoside Antibiotics. Biochem. Biophys. Res. Comm 1978, 83, 991–997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

