Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients With Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221)
- PMID: 31855498
- PMCID: PMC7062457
- DOI: 10.1200/JCO.19.01203
Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients With Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221)
Abstract
Purpose: Despite reported widespread use of dietary supplements during cancer treatment, few empirical data with regard to their safety or efficacy exist. Because of concerns that some supplements, particularly antioxidants, could reduce the cytotoxicity of chemotherapy, we conducted a prospective study ancillary to a therapeutic trial to evaluate associations between supplement use and breast cancer outcomes.
Methods: Patients with breast cancer randomly assigned to an intergroup metronomic trial of cyclophosphamide, doxorubicin, and paclitaxel were queried on their use of supplements at registration and during treatment (n =1,134). Cox proportional hazards regression adjusting for clinical and lifestyle variables was used. Recurrence and survival were indexed at 6 months after enrollment using a landmark approach.
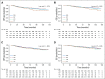
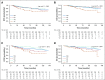
Results: There were indications that use of any antioxidant supplement (vitamins A, C, and E; carotenoids; coenzyme Q10) both before and during treatment was associated with an increased hazard of recurrence (adjusted hazard ratio [adjHR], 1.41; 95% CI, 0.98 to 2.04; P = .06) and, to a lesser extent, death (adjHR, 1.40; 95% CI, 0.90 to 2.18; P = .14). Relationships with individual antioxidants were weaker perhaps because of small numbers. For nonantioxidants, vitamin B12 use both before and during chemotherapy was significantly associated with poorer disease-free survival (adjHR, 1.83; 95% CI, 1.15 to 2.92; P < .01) and overall survival (adjHR, 2.04; 95% CI, 1.22 to 3.40; P < .01). Use of iron during chemotherapy was significantly associated with recurrence (adjHR, 1.79; 95% CI, 1.20 to 2.67; P < .01) as was use both before and during treatment (adjHR, 1.91; 95% CI, 0.98 to 3.70; P = .06). Results were similar for overall survival. Multivitamin use was not associated with survival outcomes.
Conclusion: Associations between survival outcomes and use of antioxidant and other dietary supplements both before and during chemotherapy are consistent with recommendations for caution among patients when considering the use of supplements, other than a multivitamin, during chemotherapy.
Figures
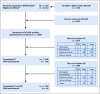
Comment in
-
Reply to E. Javor et al.J Clin Oncol. 2020 Jun 20;38(18):2111. doi: 10.1200/JCO.20.00546. Epub 2020 Apr 21. J Clin Oncol. 2020. PMID: 32315274 Free PMC article. No abstract available.
-
Dietary Supplement Use and Patient Outcomes in High-Risk Early-Stage Breast Cancer.J Clin Oncol. 2020 Jun 20;38(18):2110. doi: 10.1200/JCO.20.00091. Epub 2020 Apr 21. J Clin Oncol. 2020. PMID: 32315275 No abstract available.
References
-
- Velicer CM, Ulrich CM. Vitamin and mineral supplement use among US adults after cancer diagnosis: A systematic review. J Clin Oncol. 2008;26:665–673. - PubMed
-
- Cassileth BR, Vickers AJ. High prevalence of complementary and alternative medicine use among cancer patients: Implications for research and clinical care. J Clin Oncol. 2005;23:2590–2592. - PubMed
-
- Harvie M. Nutritional supplements and cancer: Potential benefits and proven harms. Am Soc Clin Oncol Educ Book. 2014;34:e478–e486. - PubMed
-
- Lawenda BD, Kelly KM, Ladas EJ, et al. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J Natl Cancer Inst. 2008;100:773–783. - PubMed
-
- Norman HA, Butrum RR, Feldman E, et al. The role of dietary supplements during cancer therapy. J Nutr. 2003;133:3794S–3799S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01 CA004919/CA/NCI NIH HHS/United States
- U10 CA004919/CA/NCI NIH HHS/United States
- P30 CA016056/CA/NCI NIH HHS/United States
- N01 CA013612/CA/NCI NIH HHS/United States
- R01 CA116395/CA/NCI NIH HHS/United States
- U10 CA180801/CA/NCI NIH HHS/United States
- U10 CA180858/CA/NCI NIH HHS/United States
- U10 CA180828/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- U10 CA013612/CA/NCI NIH HHS/United States
- UG1 CA189974/CA/NCI NIH HHS/United States
- U10 CA046282/CA/NCI NIH HHS/United States
- U10 CA180863/CA/NCI NIH HHS/United States
- U10 CA180819/CA/NCI NIH HHS/United States
- UG1 CA233328/CA/NCI NIH HHS/United States
- R01 CA139426/CA/NCI NIH HHS/United States
- U10 CA068183/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

