Emerging roles of network analysis for epilepsy
- PMID: 31855828
- PMCID: PMC6990460
- DOI: 10.1016/j.eplepsyres.2019.106255
Emerging roles of network analysis for epilepsy
Abstract
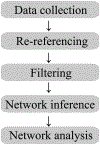
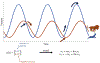
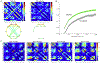
In recent years there has been increasing interest in applying network science tools to EEG data. At the 2018 American Epilepsy Society conference in New Orleans, LA, the yearly session of the Engineering and Neurostimulation Special Interest Group focused on emerging, translational technologies to analyze seizure networks. Each speaker demonstrated practical examples of how network tools can be utilized in clinical care and provide additional data to help care for patients with intractable epilepsy. The groups presented advances using tools from functional connectivity, control theory, and graph theory to analyze human EEG data. These tools have great potential to augment clinical interpretation of EEG signals.
Keywords: Control theory; EEG; Functional connectivity; Graph theory; Infantile spasms; Network analysis.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures
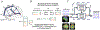
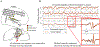
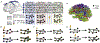
References
-
- Bowyer SM, 2016. Coherence a measure of the brain networks: past and present. Neuropsychiatric Electrophysiology 2, 1.
-
- Brazier MA, 1972. Spread of seizure discharges in epilepsy: anatomical and electrophysiological considerations. Exp Neurol 36, 263–272. - PubMed

