Renal Morbidity of 6% Hydroxyethyl Starch 130/0.4 in 9000 Propensity Score Matched Pairs of Surgical Patients
- PMID: 31856004
- PMCID: PMC7249485
- DOI: 10.1213/ANE.0000000000004592
Renal Morbidity of 6% Hydroxyethyl Starch 130/0.4 in 9000 Propensity Score Matched Pairs of Surgical Patients
Abstract
Background: Several studies of critically ill patients reported that fluid resuscitation with hydroxyethyl starch (HES) solutions damages the kidneys, but their use for surgical patients is debated. Because different HES preparations have different safety profiles, we sought to determine whether 6% third-generation HES 130/0.4 was associated with renal morbidity when used for surgical patients.
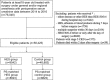
Methods: We identified adults enrolled in a Japanese nationwide medical database who underwent surgery between 2014 and 2016, with HES 130/0.4 or without it (controls). These groups were balanced with propensity score matching in a 1:1 ratio without replacement by multivariable logistic regression with 36 covariates, including demographic characteristics, preoperative comorbidities, and anesthetic/surgical procedures. The primary outcome was the incidence of acute kidney injury (AKI) in patients receiving intraoperative HES and controls. Secondary outcomes were assessing whether HES was associated with worsening AKI stage, the incidence of renal-replacement therapy (RRT), hospital length-of-stay, and in-hospital 30-day mortality. Tertiary outcomes include the use of vasoactive agents and the fluid requirement on the day of surgery. Comparative analysis was made with χ, Mann-Whitney U test, or the ordinal logistic regression analysis.
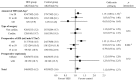
Results: Of 76,048 patients in the database, 58,425 were eligible: 9542 received HES and 48,883 controls. Propensity score matching identified 8823 matched pairs. The incidence of AKI was 6.2% (548/8823) in the HES group and 5.6% (492/8823) in controls (odds ratio [OR], 1.12; 95% confidence interval [CI], 0.99-1.27; P = .07). Compared to controls, HES was not associated with worsening AKI stage (OR, 0.89; 95% CI, 0.79-1.01; P = .08). The incidence of RRT was lower in the HES group than that in controls (0.2% vs 0.4%, respectively; OR, 0.51; 95% CI, 0.29-0.91; P = .02). Median [interquartile range] hospital stay was 1 day longer in the HES group (12 [8-21] vs 11 [7-20] days; P < .001), but in-hospital 30-day mortality did not differ between groups (0.5% vs 0.6%, respectively: OR, 0.83; 95% CI, 0.56-1.24; P = .36). The use rate of vasoactive agents and the median net fluid requirement on the day of surgery were higher in the HES group (80.5% vs 70.0%: P < .001, 88.1 vs 73.6 mL/kg; P < .001, respectively) compared to controls.
Conclusions: The present study did not demonstrate that 6% HES 130/0.4 increased the incidence and the severity of postoperative AKI. It was associated with a lower incidence of RRT when used for surgical patients.
Conflict of interest statement
Conflicts of Interest: See Disclosures at the end of the article.
Figures
Comment in
-
Propensity Score Matching in Observational Research.Anesth Analg. 2020 Jun;130(6):1616-1617. doi: 10.1213/ANE.0000000000004770. Anesth Analg. 2020. PMID: 32384349 No abstract available.
-
Meet the New Hydroxyethyl Starch, Same as the Old Hydroxyethyl Starch?Anesth Analg. 2020 Aug;131(2):e87-e88. doi: 10.1213/ANE.0000000000004916. Anesth Analg. 2020. PMID: 33031682 No abstract available.
-
In Response.Anesth Analg. 2020 Aug;131(2):e88-e90. doi: 10.1213/ANE.0000000000004917. Anesth Analg. 2020. PMID: 33031683 No abstract available.
References
-
- Brunkhorst FM, Engel C, Bloos F, et al. ; German Competence Network Sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. - PubMed
-
- Perner A, Haase N, Guttormsen AB, et al. ; 6S Trial Group; Scandinavian Critical Care Trials Group. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367:124–134. - PubMed
-
- Myburgh JA, Finfer S, Bellomo R, et al. ; CHEST Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367:1901–1911. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

