Childhood immune imprinting to influenza A shapes birth year-specific risk during seasonal H1N1 and H3N2 epidemics
- PMID: 31856206
- PMCID: PMC6922319
- DOI: 10.1371/journal.ppat.1008109
Childhood immune imprinting to influenza A shapes birth year-specific risk during seasonal H1N1 and H3N2 epidemics
Abstract
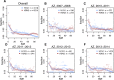
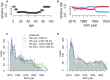
Across decades of co-circulation in humans, influenza A subtypes H1N1 and H3N2 have caused seasonal epidemics characterized by different age distributions of cases and mortality. H3N2 causes the majority of severe, clinically attended cases in high-risk elderly cohorts, and the majority of overall deaths, whereas H1N1 causes fewer deaths overall, and cases shifted towards young and middle-aged adults. These contrasting age profiles may result from differences in childhood imprinting to H1N1 and H3N2 or from differences in evolutionary rate between subtypes. Here we analyze a large epidemiological surveillance dataset to test whether childhood immune imprinting shapes seasonal influenza epidemiology, and if so, whether it acts primarily via homosubtypic immune memory or via broader, heterosubtypic memory. We also test the impact of evolutionary differences between influenza subtypes on age distributions of cases. Likelihood-based model comparison shows that narrow, within-subtype imprinting shapes seasonal influenza risk alongside age-specific risk factors. The data do not support a strong effect of evolutionary rate, or of broadly protective imprinting that acts across subtypes. Our findings emphasize that childhood exposures can imprint a lifelong immunological bias toward particular influenza subtypes, and that these cohort-specific biases shape epidemic age distributions. As a consequence, newer and less "senior" antibody responses acquired later in life do not provide the same strength of protection as responses imprinted in childhood. Finally, we project that the relatively low mortality burden of H1N1 may increase in the coming decades, as cohorts that lack H1N1-specific imprinting eventually reach old age.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Francis T. On the Doctrine of Original Antigenic Sin. Proc Am Philos Soc. 1960;104: 572–578.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

