Longitudinal assessments of plasma ADAMTS13 biomarkers predict recurrence of immune thrombotic thrombocytopenic purpura
- PMID: 31856267
- PMCID: PMC6929391
- DOI: 10.1182/bloodadvances.2019000939
Longitudinal assessments of plasma ADAMTS13 biomarkers predict recurrence of immune thrombotic thrombocytopenic purpura
Abstract
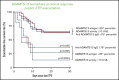
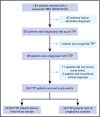
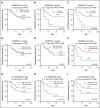
Immune thrombotic thrombocytopenic purpura (iTTP) is primarily caused by immunoglobulin G (IgG)-type autoantibodies that bind and inhibit plasma ADAMTS13 activity and/or accelerate its clearance from circulation. Approximately 50% of patients with iTTP who achieve initial clinical response to therapy experience recurrence (ie, exacerbation and/or relapse); however, a reliable biomarker that predicts such an event is currently lacking. The present study determines the role of longitudinal assessments of plasma ADAMTS13 biomarkers in predicting iTTP exacerbation/recurrence. Eighty-three unique iTTP patients with 97 episodes from the University of Alabama at Birmingham Medical Center between April 2006 and June 2019 were enrolled. Plasma levels of ADAMTS13 activity, antigen, and anti-ADAMTS13 IgG on admission showed no significant value in predicting iTTP exacerbation or recurrence. However, persistently low plasma ADAMTS13 activity (<10 U/dL; hazard ratio [HR], 4.4; 95% confidence interval [CI], 1.6-12.5; P = .005) or high anti-ADAMTS13 IgG (HR, 3.1; 95% CI, 1.2-7.8; P = .016) 3 to 7 days after the initiation of therapeutic plasma exchange was associated with an increased risk for exacerbation or recurrence. Furthermore, low plasma ADAMTS13 activity (<10 IU/dL; HR, 4.8; 95% CI, 1.8-12.8; P = .002) and low ADAMTS13 antigen (<25th percentile; HR, 3.3; 95% CI, 1.3-8.2; P = .01) or high anti-ADAMTS13 IgG (>75th percentile; HR, 2.6; 95% CI, 1.0-6.5; P = .047) at clinical response or remission was also predictive of exacerbation or recurrence. Our results suggest the potential need for a more aggressive approach to achieve biochemical remission (ie, normalization of plasma ADAMTS13 activity, ADAMTS13 antigen, and anti-ADAMTS13 IgG) in patients with iTTP to prevent the disease recurrence.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: X.L.Z. is a speaker or consultant for Alexion, Ablynx/Sanofi, and Shire/Takeda and is also the founder of Clotsolution, Inc. The remaining authors declare no competing financial interests.
Figures


References
-
- Bell WR, Braine HG, Ness PM, Kickler TS. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience in 108 patients. N Engl J Med. 1991;325(6):398-403. - PubMed
-
- Rock GA, Shumak KH, Buskard NA, et al. ; Canadian Apheresis Study Group . Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N Engl J Med. 1991;325(6):393-397. - PubMed
-
- Lindblom A, Thorsen S, Hillarp A, Björk P. Minor stroke as singular manifestation of hereditary thrombotic thrombocytopenic purpura in a young man. Int Angiol. 2009;28(4):336-339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

