Regorafenib plus FOLFIRI with irinotecan dose escalated according to uridine diphosphate glucuronosyltransferase 1A1genotyping in previous treated metastatic colorectal cancer patients:study protocol for a randomized controlled trial
- PMID: 31856912
- PMCID: PMC6923824
- DOI: 10.1186/s13063-019-3917-z
Regorafenib plus FOLFIRI with irinotecan dose escalated according to uridine diphosphate glucuronosyltransferase 1A1genotyping in previous treated metastatic colorectal cancer patients:study protocol for a randomized controlled trial
Abstract
Background: Regorafenib is an oral multikinase inhibitor for metastatic colorectal cancer (mCRC) previously treated with fluoropyrimidines, irinotecan, oxaliplatin, monoclonal antibodies targeting vascular endothelial growth factor, and monoclonal antibodies targeting epidermal growth factor receptor. A dose reduction from 160 mg to 120 mg regorafenib reduces regorafenib-associated adverse events (AEs). Dose adjustment of irinotecan in a 5-fluorouracil/leucovorin/irinotecan (FOLFIRI) regimen on the basis of an individual uridine diphosphate glucuronosyl transferase 1A1 (UGT1A1) genotype provides optimal oncological outcomes with acceptable AEs. The aim of this study is to address the efficacy and safety of a dose-adjusted combination of regorafenib and FOLFIRI for patients with mCRC.
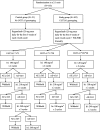
Methods: A prospective, multicenter, randomized in a 2:1 ratio, controlled, clinical trial with two parallel arms will be conducted to compare irinotecan dose-escalated FOLFIRI according to UGT1A1 genotyping plus 120 mg regorafenib with 120 mg regorafenib alone in previously treated patients with mCRC. The primary endpoint is progression-free survival, and the secondary endpoints are overall survival, disease control rate, time to progression, and duration of treatment. Safety assessments will also be recorded.
Discussion: Dose adjustment for regorafenib and irinotecan makes treatment-related AEs tolerable and makes the concomitant treatment practicable. This study will provide initial evidence regarding the efficacy and safety of a new combination of chemotherapy and a targeted agent for mCRC.
Trial registration: ClinicalTrials.gov, NCT03880877. Prospectively registered on 19 March 2019.
Keywords: Dose escalation; FOLFIRI; Metastatic colorectal cancer; Regorafenib; UGT1A1.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Regorafenib Plus FOLFIRI With Irinotecan Dose Escalated According to Uridine Diphosphate Glucuronosyltransferase 1A1 Genotyping in Patients With Metastatic Colorectal Cancer.Oncol Res. 2017 May 24;25(5):673-679. doi: 10.3727/97818823455816X14786040691928. Epub 2016 Nov 17. Oncol Res. 2017. PMID: 27938508 Free PMC article.
-
Determination of the UGT1A1 polymorphism as guidance for irinotecan dose escalation in metastatic colorectal cancer treated with first-line bevacizumab and FOLFIRI (PURE FIST).Eur J Cancer. 2020 Oct;138:19-29. doi: 10.1016/j.ejca.2020.05.031. Epub 2020 Aug 20. Eur J Cancer. 2020. PMID: 32829105 Clinical Trial.
-
FOLFIRINOX-R study design: a phase I/II trial of FOLFIRINOX plus regorafenib as first line therapy in patients with unresectable RAS-mutated metastatic colorectal cancer.BMC Cancer. 2021 May 17;21(1):564. doi: 10.1186/s12885-021-08312-7. BMC Cancer. 2021. PMID: 34001059 Free PMC article.
-
Sequencing of antiangiogenic agents in the treatment of metastatic colorectal cancer.Clin Colorectal Cancer. 2014 Sep;13(3):135-44. doi: 10.1016/j.clcc.2014.02.001. Epub 2014 Feb 27. Clin Colorectal Cancer. 2014. PMID: 24768040 Review.
-
[Regorafenib in patients with metastatic colorectal cancer: a review and an update].Recenti Prog Med. 2015 Dec;106(12):629-33. doi: 10.1701/2094.22654. Recenti Prog Med. 2015. PMID: 26780072 Review. Italian.
Cited by
-
Association of UGT1A1*6 polymorphism with irinotecan-based chemotherapy reaction in colorectal cancer patients: a systematic review and a meta-analysis.Biosci Rep. 2020 Oct 30;40(10):BSR20200576. doi: 10.1042/BSR20200576. Biosci Rep. 2020. PMID: 32936306 Free PMC article.
-
Characteristics and Clinical Implication of UGT1A1 Heterozygous Mutation in Tumor.Zhongguo Fei Ai Za Zhi. 2022 Mar 20;25(3):137-146. doi: 10.3779/j.issn.1009-3419.2022.101.07. Zhongguo Fei Ai Za Zhi. 2022. PMID: 35340156 Free PMC article.
-
UGT1A1 Guided Cancer Therapy: Review of the Evidence and Considerations for Clinical Implementation.Cancers (Basel). 2021 Mar 29;13(7):1566. doi: 10.3390/cancers13071566. Cancers (Basel). 2021. PMID: 33805415 Free PMC article. Review.
References
-
- Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schutz G, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129(1):245–255. doi: 10.1002/ijc.25864. - DOI - PubMed
-
- Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–312. doi: 10.1016/S0140-6736(12)61900-X. - DOI - PubMed
-
- Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16(6):619–629. doi: 10.1016/S1470-2045(15)70156-7. - DOI - PubMed
-
- Mathijssen RH, van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G, et al. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11) Clin Cancer Res. 2001;7(8):2182–2194. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials