Chimeric Antigen Receptor T-Cell Therapy for Cancer and Heart: JACC Council Perspectives
- PMID: 31856973
- PMCID: PMC8211027
- DOI: 10.1016/j.jacc.2019.10.049
Chimeric Antigen Receptor T-Cell Therapy for Cancer and Heart: JACC Council Perspectives
Abstract
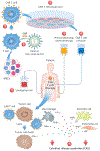
Chimeric antigen receptor (CAR) T-cell therapy has significantly advanced the treatment of patients with relapsed and refractory hematologic malignancies and is increasingly investigated as a therapeutic option of other malignancies. The main adverse effect of CAR T-cell therapy is potentially life-threatening cytokine release syndrome (CRS). Clinical cardiovascular (CV) manifestations of CRS include tachycardia, hypotension, troponin elevation, reduced left ventricular ejection fraction, pulmonary edema, and cardiogenic shock. Although insults related to CRS toxicity might be transient and reversible in most instances in patients with adequate CV reserve, they can be particularly challenging in higher-risk, often elderly patients with pre-existing CV disease. As the use of CAR T-cell therapy expands to include a wider patient population, careful patient selection, pre-treatment cardiac evaluation, and CV risk stratification should be considered within the CAR T-cell treatment protocol. Early diagnosis and management of CV complications in patients with CRS require awareness and multidisciplinary collaboration.
Keywords: cardiotoxicity; chimeric antigen receptor T-cell therapy; cytokine release syndrome.
Copyright © 2019 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

