Comparison between electronic and paper versions of patient-reported outcome measures in subjects with chronic obstructive pulmonary disease: an observational study with a cross-over administration
- PMID: 31857313
- PMCID: PMC6937099
- DOI: 10.1136/bmjopen-2019-032767
Comparison between electronic and paper versions of patient-reported outcome measures in subjects with chronic obstructive pulmonary disease: an observational study with a cross-over administration
Abstract
Objectives: A wide range of electronic devices can be used for data collection of patient-reported outcome (PRO) measures in subjects with chronic obstructive pulmonary disease (COPD). Although comparisons between electronic and paper-based PRO measures have been undertaken in asthmatics, it is currently uncertain whether electronic questionnaires work equally as well as paper versions in elderly subjects with COPD. The aim of this study was to compare the responses to paper and electronic versions of the Evaluating Respiratory Symptoms in COPD (E-RS) and the COPD Assessment Test (CAT).
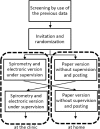
Design: A randomised cross-over design was used to compare the responses to paper and electronic versions of the two tools. The interval between the two administrations was 1 week.
Setting: Electronic versions were self-administered under supervision using a tablet computer at our outpatient clinic (secondary care hospital in Japan) while paper questionnaires completed at home were requested to be returned by mail. It was intended that half of the patients completed the electronic versions of both questionnaires first, followed by the paper versions while the other half completed the paper versions first.
Participants: Eighty-one subjects with stable COPD were included.
Results: The E-RS total scores (possible range 0-40) were 6.8±7.4 and 5.0±6.6 in the paper-based and electronic versions, respectively, and the CAT scores (possible range 0-40) were 10.0±7.4 and 8.6±7.8. In both questionnaires, higher scores indicate worse status. The relationship between electronic and paper versions showed significant reliability for both the E-RS total score and CAT score (intraclass correlation coefficient=0.82 and 0.89, respectively; both p<0.001). However, both the E-RS total and CAT scores were significantly higher in the paper versions (p<0.05).
Conclusions: In both cases, the two versions of the same questionnaire cannot be used interchangeably even though they have both been validated.
Keywords: chronic obstructive pulmonary disease (COPD); health status; patient-reported outcome (PRO); the COPD assessment test (CAT); the evaluating respiratory symptoms in COPD (E-RS).
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous