Response Assessment in Neuro-Oncology Criteria for Gliomas: Practical Approach Using Conventional and Advanced Techniques
- PMID: 31857322
- PMCID: PMC6975322
- DOI: 10.3174/ajnr.A6358
Response Assessment in Neuro-Oncology Criteria for Gliomas: Practical Approach Using Conventional and Advanced Techniques
Abstract
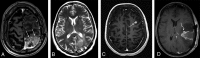
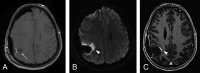
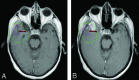
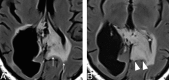
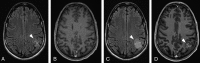
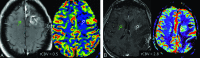
The Response Assessment in Neuro-Oncology criteria were developed as an objective tool for radiologic assessment of treatment response in high-grade gliomas. Imaging plays a critical role in the management of the patient with glioma, from initial diagnosis to posttreatment follow-up, which can be particularly challenging for radiologists. Interpreting findings after surgery, radiation, and chemotherapy requires profound knowledge about the tumor biology, as well as the peculiar changes expected to ensue as a consequence of each treatment technique. In this article, we discuss the imaging findings associated with tumor progression, tumor response, pseudoprogression, and pseudoresponse according to the Response Assessment in Neuro-Oncology criteria for high-grade and lower-grade gliomas. We describe relevant practical issues when evaluating patients with glioma, such as the need for imaging in the first 48 hours, the radiation therapy planning and isodose curves, the significance of T2/FLAIR hyperintense lesions, the impact of the timing for the evaluation after radiation therapy, and the definition of progressive disease on the histologic specimen. We also illustrate the correlation among the findings on conventional MR imaging with advanced techniques, such as perfusion, diffusion-weighted imaging, spectroscopy, and amino acid PET. Because many of the new lesions represent a mixture of tumor cells and tissue with radiation injury, the radiologist aims to identify the predominant component of the lesion and categorize the findings according to Response Assessment in Neuro-Oncology criteria so that the patient can receive the best treatment.
© 2020 by American Journal of Neuroradiology.
Figures




References
-
- Ostrom QT, Gittleman H, Stetson L, et al. . Epidemiology of intracranial gliomas In: Intracranial Gliomas: Part I-Surgery. Vol 30 Basel: Karger Publishers; 2018:1–11 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
