Mutation-derived Neoantigen-specific T-cell Responses in Multiple Myeloma
- PMID: 31857430
- PMCID: PMC6980765
- DOI: 10.1158/1078-0432.CCR-19-2309
Mutation-derived Neoantigen-specific T-cell Responses in Multiple Myeloma
Abstract
Purpose: Somatic mutations in cancer cells can give rise to novel protein sequences that can be presented by antigen-presenting cells as neoantigens to the host immune system. Tumor neoantigens represent excellent targets for immunotherapy, due to their specific expression in cancer tissue. Despite the widespread use of immunomodulatory drugs and immunotherapies that recharge T and NK cells, there has been no direct evidence that neoantigen-specific T-cell responses are elicited in multiple myeloma.
Experimental design: Using next-generation sequencing data we describe the landscape of neo-antigens in 184 patients with multiple myeloma and successfully validate neoantigen-specific T cells in patients with multiple myeloma and support the feasibility of neoantigen-based therapeutic vaccines for use in cancers with intermediate mutational loads such as multiple myeloma.
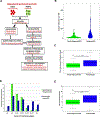
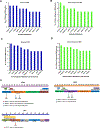
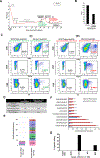
Results: In this study, we demonstrate an increase in neoantigen load in relapsed patients with multiple myeloma as compared with newly diagnosed patients with multiple myeloma. Moreover, we identify shared neoantigens across multiple patients in three multiple myeloma oncogenic driver genes (KRAS, NRAS, and IRF4). Next, we validate neoantigen T-cell response and clonal expansion in correlation with clinical response in relapsed patients with multiple myeloma. This is the first study to experimentally validate the immunogenicity of predicted neoantigens from next-generation sequencing in relapsed patients with multiple myeloma.
Conclusions: Our findings demonstrate that somatic mutations in multiple myeloma can be immunogenic and induce neoantigen-specific T-cell activation that is associated with antitumor activity in vitro and clinical response in vivo. Our results provide the foundation for using neoantigen targeting strategies such as peptide vaccines in future trials for patients with multiple myeloma.
©2019 American Association for Cancer Research.
Figures



References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: A Cancer Journal for Clinicians 2016;66(1):7–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

