Macrophages in cardiac repair: Environmental cues and therapeutic strategies
- PMID: 31857583
- PMCID: PMC6923399
- DOI: 10.1038/s12276-019-0269-4
Macrophages in cardiac repair: Environmental cues and therapeutic strategies
Abstract
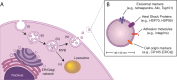
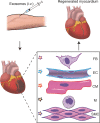
Mammals, in contrast to urodeles and teleost fish, lose the ability to regenerate their hearts soon after birth. Central to this regenerative response are cardiac macrophages, which comprise a heterogeneous population of cells with origins from the yolk sac, fetal liver, and bone marrow. These cardiac macrophages maintain residency in the myocardium through local proliferation and partial replacement over time by circulating monocytes. The intrinsic plasticity of cardiac macrophages in the adult heart promotes dynamic phenotypic changes in response to environmental cues, which may either protect against injury or promote maladaptive remodeling. Thus, therapeutic strategies promoting myocardial repair are warranted. Adult stromal cell-derived exosomes have shown therapeutic promise by skewing macrophages toward a cardioprotective phenotype. While several key exosomal non-coding RNA have been identified, additional factors responsible for cardiomyocyte proliferation remain to be elucidated. Here I review cardiac macrophages in development and following injury, unravel environmental cues modulating macrophage activation, and assess novel approaches for targeted delivery.
Conflict of interest statement
G. de Couto is a paid consultant for Capricor, Inc. Capricor neither provided funding for this work nor did the company have approval rights over the manuscript.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

