Auxin-induced actin cytoskeleton rearrangements require AUX1
- PMID: 31859367
- PMCID: PMC7154765
- DOI: 10.1111/nph.16382
Auxin-induced actin cytoskeleton rearrangements require AUX1
Abstract
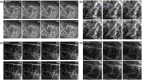
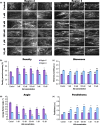
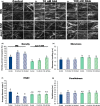
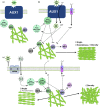
The actin cytoskeleton is required for cell expansion and implicated in cellular responses to the phytohormone auxin. However, the mechanisms that coordinate auxin signaling, cytoskeletal remodeling and cell expansion are poorly understood. Previous studies examined long-term actin cytoskeleton responses to auxin, but plants respond to auxin within minutes. Before this work, an extracellular auxin receptor - rather than the auxin transporter AUXIN RESISTANT 1 (AUX1) - was considered to precede auxin-induced cytoskeleton reorganization. In order to correlate actin array organization and dynamics with degree of cell expansion, quantitative imaging tools established baseline actin organization and illuminated individual filament behaviors in root epidermal cells under control conditions and after indole-3-acetic acid (IAA) application. We evaluated aux1 mutant actin organization responses to IAA and the membrane-permeable auxin 1-naphthylacetic acid (NAA). Cell length predicted actin organization and dynamics in control roots; short-term IAA treatments stimulated denser and more parallel, longitudinal arrays by inducing filament unbundling within minutes. Although AUX1 is necessary for full actin rearrangements in response to auxin, cytoplasmic auxin (i.e. NAA) stimulated a lesser response. Actin filaments became more 'organized' after IAA stopped elongation, refuting the hypothesis that 'more organized' actin arrays universally correlate with rapid growth. Short-term actin cytoskeleton response to auxin requires AUX1 and/or cytoplasmic auxin.
Keywords: AUX1; Arabidopsis; actin; auxin; cell expansion; cytoskeleton; signaling.
© 2019 The Authors. New Phytologist © 2019 New Phytologist Trust.
Figures


References
-
- Andrianantoandro E, Blanchoin L, Sept D, McCammon JA, Pollard TD. 2001. Kinetic mechanism of end‐to‐end annealing of actin filaments. Journal of Molecular Biology 312: 721–730. - PubMed
-
- Baluška F, Jasik J, Edelmann HG, Salajová T, Volkmann D. 2001. Latrunculin B‐induced plant dwarfism: plant cell elongation is F‐actin‐dependent. Developmental Biology 231: 113–124. - PubMed
-
- Baluška F, Vitha S, Barlow PW, Volkmann D. 1997. Rearrangements of F‐actin arrays in growing cells of intact maize root apex tissues: a major developmental switch occurs in the postmitotic transition region. European Journal of Cell Biology 72: 113–121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

