Association of Apolipoprotein E ε4 With Medial Temporal Tau Independent of Amyloid-β
- PMID: 31860000
- PMCID: PMC6990684
- DOI: 10.1001/jamaneurol.2019.4421
Association of Apolipoprotein E ε4 With Medial Temporal Tau Independent of Amyloid-β
Abstract
Importance: Apolipoprotein E ε4 (APOEε4) is the single most important genetic risk factor for Alzheimer disease. While APOEε4 is associated with increased amyloid-β burden, its association with cerebral tau pathology has been controversial.
Objective: To determine whether APOEε4 is associated with medial temporal tau pathology independently of amyloid-β, sex, clinical status, and age.
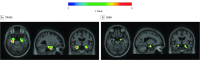
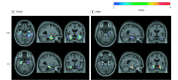
Design, setting, and participants: This is a study of 2 cross-sectional cohorts of volunteers who were cognitively normal, had mild cognitive impairment (MCI), or had Alzheimer disease dementia: the Translational Biomarkers in Aging and Dementia (TRIAD) study (data collected between October 2017 and July 2019) and the Alzheimer's Disease Neuroimaging Initiative (ADNI) (collected between November 2015 and June 2019). The first cohort (TRIAD) comprised cognitively normal elderly participants (n = 124), participants with MCI (n = 50), and participants with Alzheimer disease (n = 50) who underwent tau positron emission tomography (PET) with fluorine 18-labeled MK6240 and amyloid-β PET with [18F]AZD4694. The second sample (ADNI) was composed of cognitively normal elderly participants (n = 157), participants with MCI (n = 83), and participants with Alzheimer disease (n = 25) who underwent tau PET with [18F]flortaucipir and amyloid-β PET with [18F]florbetapir. Exclusion criteria were a history of other neurological disorders, stroke, or head trauma. There were 489 eligible participants, selected based on availability of amyloid-PET, tau-PET, magnetic resonance imaging, and genotyping for APOEε4. Forty-five young adults (<30 years) from the TRIAD cohort were not selected for this study.
Main outcomes and measures: A main association between APOEε4 and tau-PET standardized uptake value ratio, correcting for age, sex, clinical status, and neocortical amyloid-PET standardized uptake value ratio.
Results: The mean (SD) age of the 489 participants was 70.5 (7.1) years; 171 were APOEε4 carriers (34.9%), and 230 of 489 were men. In both cohorts, APOEε4 was associated in increased tau-PET uptake in the entorhinal cortex and hippocampus independently of amyloid-β, sex, age, and clinical status after multiple comparisons correction (TRIAD: β = 0.33; 95% CI, 0.19-0.49; ADNI: β = 0.13; 95% CI, 0.08-0.19; P < .001).
Conclusions and relevance: Our results indicate that the elevated risk of developing dementia conferred by APOEε4 genotype involves mechanisms associated with both amyloid-β and tau aggregation. These results contribute to an evolving framework in which APOEε4 has deleterious consequences in Alzheimer disease beyond its link with amyloid-β and suggest APOEε4 as a potential target for future disease-modifying therapeutic trials targeting tau pathology.
Conflict of interest statement
Figures

References
-
- Farrer LA, Cupples LA, Haines JL, et al. ; APOE and Alzheimer Disease Meta Analysis Consortium . Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278(16):1349-1356. doi:10.1001/jama.1997.03550160069041 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

