Determining Levers of Cost-effectiveness for Screening Infants at High Risk for Peanut Sensitization Before Early Peanut Introduction
- PMID: 31860109
- PMCID: PMC6991237
- DOI: 10.1001/jamanetworkopen.2019.18041
Determining Levers of Cost-effectiveness for Screening Infants at High Risk for Peanut Sensitization Before Early Peanut Introduction
Abstract
Importance: Early peanut introduction reduces the risk of developing peanut allergy, especially in high-risk infants. Current US recommendations endorse screening but are not cost-effective relative to other international strategies.
Objective: To identify scenarios in which current early peanut introduction guidelines would be cost-effective.
Design, setting, and participants: This simulation/cohort economic evaluation used microsimulations and cohort analyses in a Markov model to evaluate the cost-effectiveness of early peanut introduction with and without peanut skin prick test (SPT) screening in high-risk infants during an 80-year horizon from a societal perspective. Data were analyzed from April to May 2019.
Exposures: High-risk infants with early-onset eczema and/or egg allergy underwent early peanut introduction with and without peanut SPT screening (100 000 infants per treatment strategy) using a dichotomous 8-mm SPT cutoff value (stipulated in the current US guideline).
Main outcomes and measures: Cost, quality-adjusted life-years (QALYs), net monetary benefit, peanut allergic reactions, severe allergic reactions, and deaths due to peanut allergy.
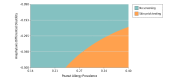
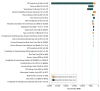
Results: In the simulated cohort of 200 000 infants and using the base case during the model horizon, a no-screening approach had lower mean (SD) costs ($13 449 [$38 163] vs $15 279 [$38 995]) and higher mean (SD) gain in QALYs (29.25 [3.28] vs 29.23 [3.30]) vs screening but resulted in more allergic reactions (mean [SD], 1.07 [3.15] vs 1.01 [3.02]), severe allergic reactions (mean [SD], 0.53 [1.66] vs 0.52 [1.62]), and anaphylaxis involving cardiorespiratory compromise (mean [SD], 0.50 [1.59] vs 0.49 [1.47]) per individual. In deterministic SPT sensitivity analyses at base-case sensitivity and specificity rates, screening could be cost-effective at a high disutility rate (the negative effect of a food allergic reaction) (76-148 days of life traded) for an at-home vs in-clinic reaction in combination with high baseline peanut allergy prevalence among infants at high risk for peanut allergy and not yet exposed to peanuts. If an equivalent rate and disutility of accidental and index anaphylaxis was assumed and the 8-mm SPT cutoff had 0.85 sensitivity and 0.98 specificity, screening was cost-effective at a peanut allergy prevalence of 36%.
Conclusions and relevance: The results of this study suggest that the current screening approach to early peanut introduction could be cost-effective at a particular health utility for an in-clinic reaction, SPT sensitivity and specificity, and high baseline peanut allergy prevalence among high-risk infants. However, such conditions are unlikely to be plausible to realistically achieve. Further research is needed to define the health state utility associated with reaction location.
Conflict of interest statement
Figures

References
-
- Oria MP, Stallings VA, eds. Finding a Path to Safety in Food Allergy: Assessment of the Global Burden, Causes, Prevention, Management, and Public Policy. Washington, DC: National Academies Press; 2016. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

