Current intensity- and polarity-specific online and aftereffects of transcranial direct current stimulation: An fMRI study
- PMID: 31860160
- PMCID: PMC7267945
- DOI: 10.1002/hbm.24901
Current intensity- and polarity-specific online and aftereffects of transcranial direct current stimulation: An fMRI study
Abstract
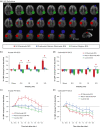
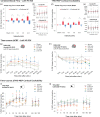
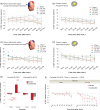
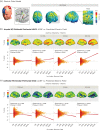
Transcranial direct current stimulation (tDCS) induces polarity- and dose-dependent neuroplastic aftereffects on cortical excitability and cortical activity, as demonstrated by transcranial magnetic stimulation (TMS) and functional imaging (fMRI) studies. However, lacking systematic comparative studies between stimulation-induced changes in cortical excitability obtained from TMS, and cortical neurovascular activity obtained from fMRI, prevent the extrapolation of respective physiological and mechanistic bases. We investigated polarity- and intensity-dependent effects of tDCS on cerebral blood flow (CBF) using resting-state arterial spin labeling (ASL-MRI), and compared the respective changes to TMS-induced cortical excitability (amplitudes of motor evoked potentials, MEP) in separate sessions within the same subjects (n = 29). Fifteen minutes of sham, 0.5, 1.0, 1.5, and 2.0-mA anodal or cathodal tDCS was applied over the left primary motor cortex (M1) in a randomized repeated-measure design. Time-course changes were measured before, during and intermittently up to 120-min after stimulation. ROI analyses indicated linear intensity- and polarity-dependent tDCS after-effects: all anodal-M1 intensities increased CBF under the M1 electrode, with 2.0-mA increasing CBF the greatest (15.3%) compared to sham, while all cathodal-M1 intensities decreased left M1 CBF from baseline, with 2.0-mA decreasing the greatest (-9.3%) from sham after 120-min. The spatial distribution of perfusion changes correlated with the predicted electric field, as simulated with finite element modeling. Moreover, tDCS-induced excitability changes correlated more strongly with perfusion changes in the left sensorimotor region compared to the targeted hand-knob region. Our findings reveal lasting tDCS-induced alterations in cerebral perfusion, which are dose-dependent with tDCS parameters, but only partially account for excitability changes.
Keywords: arterial spin labeling; cerebral blood flow; current intensity; inter-individual variability; motor cortex; transcranial direct current stimulation.
© 2019 The Authors. Human Brain Mapping published by Wiley Periodicals, Inc.
Conflict of interest statement
M.A.N. is a member of the advisory board of Neuroelectrics.
Figures


Similar articles
-
Systematic evaluation of the impact of stimulation intensity on neuroplastic after-effects induced by transcranial direct current stimulation.J Physiol. 2017 Feb 15;595(4):1273-1288. doi: 10.1113/JP272738. Epub 2016 Nov 8. J Physiol. 2017. PMID: 27723104 Free PMC article.
-
Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans.J Physiol. 2013 Apr 1;591(7):1987-2000. doi: 10.1113/jphysiol.2012.249730. Epub 2013 Jan 21. J Physiol. 2013. PMID: 23339180 Free PMC article. Clinical Trial.
-
Titrating the neuroplastic effects of cathodal transcranial direct current stimulation (tDCS) over the primary motor cortex.Cortex. 2019 Oct;119:350-361. doi: 10.1016/j.cortex.2019.04.016. Epub 2019 May 7. Cortex. 2019. PMID: 31195316
-
Searching for the optimal tDCS target for motor rehabilitation.J Neuroeng Rehabil. 2019 Jul 17;16(1):90. doi: 10.1186/s12984-019-0561-5. J Neuroeng Rehabil. 2019. PMID: 31315679 Free PMC article. Review.
-
Transcranial Direct Current Stimulation in ADHD: A Systematic Review of Efficacy, Safety, and Protocol-induced Electrical Field Modeling Results.Neurosci Bull. 2020 Oct;36(10):1191-1212. doi: 10.1007/s12264-020-00501-x. Epub 2020 May 16. Neurosci Bull. 2020. PMID: 32418073 Free PMC article.
Cited by
-
Randomized trial of cognitive training and brain stimulation in non-demented older adults.Alzheimers Dement (N Y). 2022 Feb 23;8(1):e12262. doi: 10.1002/trc2.12262. eCollection 2022. Alzheimers Dement (N Y). 2022. PMID: 35229023 Free PMC article.
-
A Literature Review on Optimal Stimulation Parameters of Transcranial Direct Current Stimulation for Motor Recovery After Stroke.Brain Neurorehabil. 2024 Nov 28;17(3):e24. doi: 10.12786/bn.2024.17.e24. eCollection 2024 Nov. Brain Neurorehabil. 2024. PMID: 39649716 Free PMC article. Review.
-
Less might be more: 1 mA but not 1.5 mA of tDCS improves tactile orientation discrimination.IBRO Neurosci Rep. 2023 Aug 21;15:186-192. doi: 10.1016/j.ibneur.2023.08.003. eCollection 2023 Dec. IBRO Neurosci Rep. 2023. PMID: 37746157 Free PMC article.
-
Effects of tDCS dose and electrode montage on regional cerebral blood flow and motor behavior.Neuroimage. 2021 Aug 15;237:118144. doi: 10.1016/j.neuroimage.2021.118144. Epub 2021 May 12. Neuroimage. 2021. PMID: 33991697 Free PMC article.
-
Variation of cerebrospinal fluid in specific regions regulates focality in transcranial direct current stimulation.Front Hum Neurosci. 2022 Sep 2;16:952602. doi: 10.3389/fnhum.2022.952602. eCollection 2022. Front Hum Neurosci. 2022. PMID: 36118967 Free PMC article.
References
-
- Alsop, D. C. , Detre, J. A. , Golay, X. , Günther, M. , Hendrikse, J. , Hernandez‐Garcia, L. , … Zaharchuk, G. (2015). Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic Resonance in Medicine, 73, 102–116. - PMC - PubMed
-
- Ambrus, G. G. , Al‐Moyed, H. , Chaieb, L. , Sarp, L. , Antal, A. , & Paulus, W. (2012). The fade‐in—short stimulation—fade out approach to sham tDCS—reliable at 1 mA for naïve and experienced subjects, but not investigators. Brain Stimulation, 5, 499–504. - PubMed
-
- Antal, A. , Terney, D. , Poreisz, C. , & Paulus, W. (2007). Towards unravelling task‐related modulations of neuroplastic changes induced in the human motor cortex. The European Journal of Neuroscience, 26, 2687–2691. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

