Intravenous iron therapy for non-anaemic, iron-deficient adults
- PMID: 31860749
- PMCID: PMC6924972
- DOI: 10.1002/14651858.CD013084.pub2
Intravenous iron therapy for non-anaemic, iron-deficient adults
Abstract
Background: Iron deficiency is one of the most common nutritional deficiencies, and has a number of physiological manifestations. Early, or non-anaemic iron deficiency can result in fatigue and diminished exercise capacity. Oral iron preparations have a high incidence of intolerable side effects, and are ineffective in certain forms of iron deficiency. Consequently, intravenous iron preparations are increasingly used in the treatment of non-anaemic iron deficiency. The newer, more stable iron preparations in particular purport to have a lower incidence of side effects, and are now used across a range of different patient populations.
Objectives: To assess the effects of intravenous iron therapy in the treatment of adults with non-anaemic iron deficiency.
Search methods: On 18 October 2019 we electronically searched CENTRAL, MEDLINE, Embase, two further databases and two trials registries 2019. We handsearched the references of full-text extracted studies, and contacted relevant study authors for additional data.
Selection criteria: We included randomised controlled trials that compared any intravenous iron preparation to placebo in adults. We excluded other forms of comparison such as oral iron versus placebo, intramuscular iron versus placebo, or intravenous iron studies where other iron preparations were used as the comparator. We also excluded studies involving erythropoietin therapy or obstetric populations.
Data collection and analysis: Two review authors screened references for eligibility, extracted data and assessed risk of bias. We resolved differences in opinion through discussion and consensus, and where necessary, involved a third review author to adjudicate disputes. We contacted study authors to request additional data where appropriate. The primary outcome measures were haemoglobin concentration at the end of follow-up, and quality-of-life scores at end of follow-up. Secondary outcome measures were serum ferritin, peak oxygen consumption (as measured by cardiopulmonary exercise testing), adverse effects (graded as mild to moderate and severe) and bacterial infection. We pooled data for continuous outcomes, which we then reported as mean differences (MDs) with 95% confidence intervals (CIs). We reported quality-of-life metrics as standardised mean difference (SMD), and then converted them back into a more familiar measure, the Piper Fatigue Scale. We analysed dichotomous outcomes as risk ratios (RRs). Given an expected degree of heterogeneity, we used a random-effects model for all outcomes. We performed the analysis with the software package Review Manager 5.
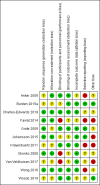
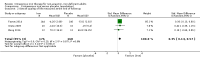
Main results: This review includes 11 studies with 1074 participants. Outcome metrics for which data were available (haemoglobin concentration, quality-of-life scores, serum ferritin, peak oxygen consumption and mild to moderate adverse effects) were similar across the included studies. The incidence of severe adverse events across all studies was zero. None of the studies measured bacterial infection as a specific outcome metric. Substantial heterogeneity influenced the results of the meta-analysis, arising from differing patient populations, definitions of iron deficiency, iron preparations and dosing regimens, and time to end of follow-up. Consequently, many outcomes are reported with small group sizes and wide confidence intervals, with a subsequent downgrading in the quality of evidence. The level of bias in many included studies was high, further reducing confidence in the robustness of the results. We found that intravenous iron therapy may lead to a small increase in haemoglobin concentration of limited clinical significance compared to placebo (MD 3.04 g/L, 95% CI 0.65 to 5.42; I2 = 42%; 8 studies, 548 participants; low-quality evidence). Quality-of-life scores (Piper Fatigue Scale MD 0.73, 95% CI 0.29 to 1.18; I2 = 0%; studies = 3) and peak oxygen consumption (MD 2.77 mL/kg/min, 95% CI -0.89 to 6.43; I2 = 36%; 2 studies, 32 participants) were associated with very low-quality evidence, and we remain uncertain about the role of intravenous iron for these metrics. We were unable to present pooled estimates for the outcomes of serum ferritin at the end of follow-up or mild to moderate adverse effects due to extreme statistical heterogeneity. Ultimately, despite the results of the meta-analysis, the low- or very low-quality evidence for all outcomes precludes any meaningful interpretation of results beyond suggesting that further research is needed. We performed a Trial Sequential Analysis for all major outcomes, none of which could be said to have reached a necessary effect size.
Authors' conclusions: Current evidence is insufficient to show benefit of intravenous iron preparations for the treatment of non-anaemic iron deficiency across a variety of patient populations, beyond stating that it may result in a small, clinically insignificant increase in haemoglobin concentration. However, the certainty for even this outcome remains limited. Robust data for the effectiveness of intravenous iron for non-anaemic iron deficiency is still lacking, and larger studies are required to assess the effect of this therapy on laboratory, patient-centric, and adverse-effect outcomes.
Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
LFM: the institution of the author has received in‐kind and unrestricted financial support for two currently running studies from Vifor Pharma Pty Ltd. The author has not personally received any honoraria or speaking fees from any company. No other conflicts of interest.
EL: the institution of the author has received in‐kind support for a study from Vifor Pharma Pty Ltd (Litton 2016). The author has not personally received any honoraria or speaking fees from any company. No other conflicts of interest.
GI: none known
DS: the institution of the author has received in‐kind and unrestricted financial support for two currently running studies from Vifor Pharma Pty Ltd. The author has not personally received any honoraria or speaking fees from any company. No other conflicts of interest.
Figures







Update of
- doi: 10.1002/14651858.CD013084
Similar articles
-
Iron for the treatment of restless legs syndrome.Cochrane Database Syst Rev. 2019 Jan 4;1(1):CD007834. doi: 10.1002/14651858.CD007834.pub3. Cochrane Database Syst Rev. 2019. PMID: 30609006 Free PMC article.
-
Interventions for treating iron deficiency anaemia in inflammatory bowel disease.Cochrane Database Syst Rev. 2021 Jan 20;1(1):CD013529. doi: 10.1002/14651858.CD013529.pub2. Cochrane Database Syst Rev. 2021. PMID: 33471939 Free PMC article.
-
Iron therapy for preoperative anaemia.Cochrane Database Syst Rev. 2019 Dec 7;12(12):CD011588. doi: 10.1002/14651858.CD011588.pub3. Cochrane Database Syst Rev. 2019. PMID: 31811820 Free PMC article.
-
Erythropoietin plus iron versus control treatment including placebo or iron for preoperative anaemic adults undergoing non-cardiac surgery.Cochrane Database Syst Rev. 2020 Aug 13;8(8):CD012451. doi: 10.1002/14651858.CD012451.pub2. Cochrane Database Syst Rev. 2020. PMID: 32790892 Free PMC article.
-
Parenteral versus oral iron therapy for adults and children with chronic kidney disease.Cochrane Database Syst Rev. 2019 Feb 21;2(2):CD007857. doi: 10.1002/14651858.CD007857.pub3. Cochrane Database Syst Rev. 2019. PMID: 30790278 Free PMC article.
Cited by
-
The need to screen for anemia in exercising women.Medicine (Baltimore). 2021 Oct 1;100(39):e27271. doi: 10.1097/MD.0000000000027271. Medicine (Baltimore). 2021. PMID: 34596123 Free PMC article.
-
Fatigue as the Chief Complaint–Epidemiology, Causes, Diagnosis, and Treatment.Dtsch Arztebl Int. 2021 Aug 23;118(33-34):566-576. doi: 10.3238/arztebl.m2021.0192. Dtsch Arztebl Int. 2021. PMID: 34196270 Free PMC article. Review.
-
The comparative effects of intravenous iron on oxidative stress and inflammation in patients with chronic kidney disease and iron deficiency: a randomized controlled pilot study.Kidney Res Clin Pract. 2021 Mar;40(1):89-98. doi: 10.23876/j.krcp.20.120. Epub 2021 Mar 22. Kidney Res Clin Pract. 2021. PMID: 33745264 Free PMC article.
-
A protocol for prospective observational study to determine if non-anaemic iron deficiency worsens postoperative outcome in adult patients undergoing elective cardiac surgery: the IDOCS study.Perioper Med (Lond). 2022 Feb 8;11(1):4. doi: 10.1186/s13741-022-00239-2. Perioper Med (Lond). 2022. PMID: 35130975 Free PMC article.
-
Preoperative patient blood management during the SARS-CoV-2 pandemic.Br J Haematol. 2021 Jun;193(6):1087-1092. doi: 10.1111/bjh.17487. Epub 2021 May 7. Br J Haematol. 2021. PMID: 33959948 Free PMC article. No abstract available.
References
References to studies included in this review
Anker 2009 {published data only (unpublished sought but not used)}
-
- Anker SD, Comin CJ, Filippatos J, Willenheimer R, Dickstein K, Drexler H, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. New England Journal of Medicine 2009;361:2436‐48. - PubMed
Burden 2015a {published data only}
-
- Burden RJ, Pollock N, Whyte GP, Richards T, Moore B, Busbridge M, et al. Effect of intravenous iron on aerobic capacity and iron metabolism in elite athletes. Medicine and Science in Sports and Exercise 2015;47(7):1399‐407. - PubMed
Charles‐Edwards 2019 {published data only}
-
- Charles‐Edwards G, Amarel N, Sleigh A, Ayis A, Catibog N, McDonagh T, et al. Effect of iron isomaltoside on skeletal muscle energetics in patients with chronic heart failure and iron deficiency ‐ FERRIC‐HF II randomized mechanistic trial. Circulation 2019;139:2386‐98. - PubMed
Favrat 2014 {published data only}
Grote 2009 {published data only}
-
- Grote L, Leissner L, Hedner J, Ulfberg J. A randomized, double‐blind, placebo controlled, multi‐center study of intravenous iron sucrose and placebo in the treatment of restless legs syndrome. Movement Disorders 2009; Vol. 24, issue 10:1445‐52. - PubMed
Johansson 2015 {published data only}
-
- Johansson PI, Rasmussen AS, Thomsen LL. Intravenous iron isomaltoside 1000 (Monofer) reduces postoperative anaemia in preoperatively non‐anaemic patients undergoing elective or subacute coronary artery bypass graft, valve replacement or a combination thereof: a randomized double‐blind placebo‐controlled clinical trial (the PROTECT trial). Vox Sanguinis 2015;109(3):257‐66. - PMC - PubMed
Krayenbuehl 2011 {published data only}
-
- Krayenbuehl PA, Battegay E, Breymann C, Furrer J, Schulthess G. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood 2011; Vol. 118, issue 12:3222‐7. - PubMed
Okonko 2008 {published data only}
-
- Okonko DO, Grzeslo A, Witkowski T, Mandal Ak, Slater RM, Roughton M, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC‐HF: a randomized, controlled, observer‐blinded trial. Journal of the American College of Cardiology 2008; Vol. 51, issue 2:103‐12. - PubMed
Van Veldhuisen 2017 {published data only}
Wong 2016 {published data only}
-
- Wong C, Ng A, Lau J, Kritharides L, Sindone A. Early responses to intravenous iron therapy in patients with chronic heart failure and iron deficiency. Heart, Lung & Circulation 2016;25 (Suppl 2):S107. - PubMed
References to studies excluded from this review
Allen 2011 {published data only}
-
- Allen RP, Butcher A, Du W. Clinical efficacy and safety of IV ferric carboxymaltose (FCM) treatment of RLS: a multi‐centred, placebo‐controlled preliminary clinical trial. Sleep Medicine 2011;12(9):906‐13. - PubMed
Arutyunov 2009 {published data only}
-
- Arutyunov GP, Bylova NA, Ivleva AY, Kobalava ZD. The safety of intravenous (IV) ferric carboxymaltose versus IV iron sucrose in patients with chronic heart failure (CHF) and chronic kidney disease (CKD) with iron deficiency (ID). European Journal of Heart Failure 2009;21(6):ii71.
Baribeault 2011 {published data only}
-
- Baribeault D. Sodium ferric gluconate (SFG) in complex with sucrose for IV infusion: bioequivalence of a new generic product with the branded product in healthy volunteers. Current Medical Research and Opinion 2011;27(8):1653‐7. - PubMed
Bart 2018 {published data only}
-
- Bart NK, Hungerford SL, Curtis MK, Cheng H‐Y, Southern JL, Petousi N, et al. Effects of modest iron loading on iron indices in healthy individuals. Journal of Applied Physiology 2018;125:1710‐19. - PubMed
Boerboom 2017 {published data only}
-
- Boerboom A, Schijns W, Aarts E, Hunfeld M, Cense H, Vrouenraets B, et al. Optimization of iron supplementation after Roux‐en‐y gastric bypass. Obesity Surgery 2017;27(Suppl 1):200.
Boomershine 2018 {published data only}
Bruinvels 2017 {published data only}
-
- Bruinvels G, Pedlar C, Burden R, Brown N, Butcher A, Chau M, et al. Ironwoman trial: the impact of intravenous iron on exercise performance in iron deficient, exercising women. American Journal of Hematology 2017;92(8):E388.
Cekic 2015 {published data only}
Cho 2016 {published data only}
-
- Cho YW, Allen RP, Earley CJ. Clinical efficacy of ferric carboxymaltose treatment in patients with restless legs syndrome. Sleep 2016;25:16‐23. - PubMed
Comin‐Colet 2013 {published data only (unpublished sought but not used)}
-
- Comin‐Colet J, Lainscak M, Dickstein K, Filippatos GS, Luscher TF, Mori C, et al. The effect of intravenous ferric carboxymaltose on health‐related quality of life in patients with chronic heart failure and iron deficiency: a subanalysis of the FAIR‐HF study. European Heart Journal 2013;34(1):30‐8. - PMC - PubMed
Deng 2017 {published data only}
Filippatos 2013 {published data only (unpublished sought but not used)}
-
- Filippatos G, Farmarkis D, Comin‐Colet JC, Dickstein K, Luscher TF, Willenheimer R, et al. Intravenous ferric carboxymaltose in iron‐deficient chronic heart failure patients with and without anaemia: a subanalysis of the FAIR‐HF trial. European Journal of Heart Failure 2013;15:1267‐76. - PMC - PubMed
Fishbane 2001 {published data only}
-
- Fishbane S, Shapiro W, Dutka P, Valenzuela OF, Faubert J. A randomized trial of iron deficiency testing strategies in hemodialysis patients. Kidney International 2001;60(6):2406‐11. - PubMed
Fontana 2014 {published data only (unpublished sought but not used)}
-
- Fontana S, Juni P, Niederhauser C, Keller P. Lack of effectiveness of intravenous iron infusion in healthy blood donors with low ferritin: a double‐blind randomized controlled trial. Vox Sanguinis 2014;107(Suppl 1):98.
Froessler 2016 {published data only}
Garvican‐Lewis 2018a {published data only}
-
- Garvican‐Lewis LA, Vuong VL, Govus AD, Peeling P, Jung G, Nemeth E, et al. Intravenous iron does not augment the hemoglobin mass response to simulated hypoxia. Medicine and Science in Sports and Exercise 2018;50(8):1669‐78. - PubMed
Garvican‐Lewis 2018b {published data only}
-
- Garvican‐Lewis LA, Vuong VL, Govus AD, Schumacher YO, Hughes D, Lovell G, et al. Influence of combined iron supplementation and simulated hypoxia on the haematological module of the athlete biological passport. Drug Testing & Analysis 2018;10(4):731‐41. - PubMed
Gutzwiller 2010 {published data only}
Gutzwiller 2012 {published data only}
-
- Gutzwiller FS, Schwenkglenks M, Blank P R, Braunhofer P G, Mori C, Szucs T D, et al. Health economic assessment of ferric carboxymaltose in patients with iron deficiency and chronic heart failure based on the FAIR‐HF trial: an analysis for the UK. European Journal of Heart Failure 2012;14(7):782‐90. - PMC - PubMed
Gybel‐Brask 2018 {published data only}
-
- Gybel‐Brask M, Seeberg J, Thomsen Ll, Johansson PI. Intravenous iron isomaltoside improves hemoglobin concentration and iron stores in female iron‐deficient blood donors: a randomized double‐blind placebo‐controlled clinical trial. Transfusion 2018;58:974‐81. - PubMed
Hsiao 2016 {published data only}
Huang 2015 {published data only}
-
- Huang L, Lee D, Kent A, Troster S, MacGinley R, Roberts MA, et al. Effects of intravenous iron carboxymaltose versus iron sucrose on fibroblast growth factor‐23 in patients receiving dialysis: a randomised controlled trial. Nephrology 2015;20(S3):45.
Kapoian 2006 {published data only}
-
- Kapoian T, Coyne DW, Rizkala AR. Safety of administration of IV iron to maintenance haemodialysis patients with elevated serum ferritin and low transferrin saturation: information from the DRIVE trial. Nephrology, Dialysis and Transplantation 2006;21(Suppl 4):iv164.
Karakas 2019 {published data only}
-
- Karakas M. Effect of ferric carboxymaltose on LVEF as assessed by cardiac MRI in patients with chronic heart failure and iron deficiency: a randomized, double‐blinded, placebo‐controlled, dual centre trial. European Journal of Heart Failure 2019;21(S1):175‐6.
Kulnigg‐Dabsch 2013 {published data only}
-
- Kulnigg‐Dabsch S, Schmid W, Howaldt S, Stein J, Mickisch O, Waldhör T, et al. Iron deficiency generates secondary thrombocytosis and platelet activation in IBD: the randomized, controlled ThromboVIT trial. Inflammatory Bowel Diseases 2013;19:1609‐16. - PubMed
Louzada 2016 {published data only}
Oliver 2010 {published data only}
-
- Oliver A, Sierra P, Noe L, Monllau V, Ortiz JC. Efficacy of post‐operative intravenous iron in elective major urological surgery. Transfusion Alternatives in Transfusion Medicine 2010;11(Suppl 2):33.
Peeling 2007 {published data only}
-
- Peeling P, Blee T, Goodman C, Dawson B, Claydon G, Beilby J, et al. Effect of iron injections on aerobic‐exercise performance of iron‐depleted female athletes. International Journal of Sport Nutrition and Exercise Metabolism 2007;17(3):221‐31. - PubMed
Ponikowski 2015 {published data only (unpublished sought but not used)}
Reinisch 2014 {published data only}
-
- Reinisch W. Intravenous iron need to maintain stable haemoglobin an open‐label, 1 year extension study of iron isomaltoside 1000 in subjects with inflammatory bowel disease. Journal of Crohn's and Colitis 2014;8(Suppl 1):S247.
Reinisch 2015 {published data only}
Rita Gomes 2018 {published data only}
-
- Rita Gomes R, Rodrigues J, Aguiar F, Graca C, Torres A, Araujo I, et al. Iron supplementation in an acute heart failure unit. European Journal of Heart Failure 2018;20(Suppl 1):94.
Sloand 2004 {published data only}
-
- Sloand JA, Shelly MA, Feigin A, Bernstein P, Monk RD. A double‐blind, placebo‐controlled trial of intravenous iron dextran therapy in patients with ESRD and restless legs syndrome. American Journal of Kidney Diseases 2004;43(4):663‐70. - PubMed
Smith 2009 {published data only}
-
- Smith TG, Talbot NP, Privat C, Rivera‐Ch M, Nickol AH, Ratcliffe PJ, et al. Effects of iron supplementation and depletion on hypoxic pulmonary hypertension: two randomized controlled trials. Journal of the American Medical Association 2009;302(13):1444‐50. - PubMed
Trenkwalder 2017 {published data only}
Van Craenenbroeck 2013 {published data only}
-
- Craenenbroeck EM, Conraads VM, Greenlaw N, Gaudesius G, Mori C, Ponikowski P, et al. The effect of intravenous ferric carboxymaltose on red cell distribution width: a subanalysis of the FAIR‐HF study. European Journal of Heart Failure 2013;15:756‐62. - PubMed
Additional references
Abbaspour 2014
Avni 2015
-
- Avni T, Bieber A, Grossman A, Green H, Leibovici L, Gafter‐Gvili A. The safety of intravenous iron preparations: systematic review and meta‐analysis. Mayo Clinic Proceedings 2015;90:12‐23. - PubMed
Bailie 2012
-
- Bailie GR. Comparison of rates of reported adverse events associated with I.V. iron products in the United States. American Journal of Health‐System Pharmacy 2012;69(4):310‐20. - PubMed
Barberan‐Garcia 2015
-
- Barberan‐Garcia A, Rodríguez DA, Blanco I, Gea J, Torralba Y, Arbillaga‐Etxarri A, et al. Non‐anaemic iron deficiency impairs response to pulmonary rehabilitation in COPD. Respirology 2015;20:1089‐95. - PubMed
Beard 2001
-
- Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. Journal of Nutrition 2001;131:S568‐80. - PubMed
Beck‐da‐Silva 2013
-
- Beck‐da‐Silva L, Piardi D, Sodler S, Rohde LE, Pereira‐Barretto AC, Albuquerque D, et al. IRON‐HF study: a randomized trial to assess the effects of iron in heart failure patients with anemia. International Journal of Cardiology 2013;168:3439‐42. - PubMed
Blaudszun 2018
-
- Blaudszun G, Munting KE, Butchart A, Gerrard C, Klein AA. The association between borderline pre‐operative anaemia in women and outcome after cardiac surgery: a cohort study. Anaesthesia 2018;73(5):572‐8. - PubMed
Burden 2015b
-
- Burden RJ, Morton K, Richards T, Whyte GP, Pedlar CR. Is iron treatment beneficial in iron‐deficient but non‐anaemic (IDNA) endurance athletes? A systematic review and meta‐analysis. British Journal of Sports Medicine 2015;49:1389‐97. - PubMed
Butcher 2017
-
- Butcher A, Richards T, Stanworth SJ, Klein AA. Diagnostic criteria for pre‐operative anaemia–time to end sex discrimination. Anaesthesia 2017;72:811‐4. - PubMed
Cancelo‐Hidalgo 2013
-
- Cancelo‐Hidalgo MJ, Castelo‐Branco C, Palacios S, Haya‐Palazuelos J, Ciria‐Recasens M, Manasanch J, et al. Tolerability of different oral iron supplements: a systematic review. Current Medical Research and Opinion 2013;29(4):291‐303. - PubMed
Cançado 2011
Clevenger 2015
-
- Clevenger B, Mallett SV, Klein AA, Richards T. Patient blood management to reduce surgical risk. British Journal of Surgery 2015;102:1325‐37. - PubMed
Copenhagen Trial Unit 2016 [Computer program]
-
- Copenhagen Trial Unit, Centre for Clinical Intervention Research. Trial Sequential Analysis. Version 0.9.5.5 Beta. Copenhagen, Denmark: Copenhagen Trial Unit, Centre for Clinical Intervention Research, 2016.
Drakesmith 2012
-
- Drakesmith H, Prentice AM. Hepcidin and the iron‐infection axis. Science 2012;338:768‐72. - PubMed
Egger 1997
Ganz 2003
-
- Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anaemia of inflammation. Blood 2003;102:783‐8. - PubMed
Ganz 2012
Gereklioglu 2016
Goodnough 2011
-
- Goodnough LT, Nemeth E, Ganz T. Detection, evaluation and management of iron‐restricted erythropoesis. Network 2011;116:4754‐61. - PubMed
Goodnough 2012
-
- Goodnough LT. Iron deficiency syndromes and iron‐restricted erythropoesis. Transfusion 2012;52:1584‐92. - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 14 January 2019. Hamilton (ON): McMaster University (developed by Evidence Prime).
Higgins 2003
Higgins 2019a
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. - PMC - PubMed
Higgins 2019b
-
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hinton 2014
-
- Hinton PS. Iron and the endurance athlete. Applied Physiology, Nutrition and Metabolism 2014;39:1012‐8. - PubMed
Houston 2018
ICH‐GCP 1996
-
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Code of Federal Regulation and ICH Guidelines. Parexel Barnett: Media, 1996.
Imberger 2015
-
- Imberger G, Gluud C, Boylan J, Wetterslev J. Systematic reviews of anesthesiologic interventions reported as statistically significant: problems with power, precision, and type 1 error protection. Anesthesia and Analgesia 2015;121:1611‐22. - PubMed
Jankowska 2016
-
- Jankowska EA, Tkaczyszyn M, Suchocki T, Drozd M, Haehling S, Doehner W, et al. Effects of intravenous iron therapy in iron‐deficient patients with systolic heart failure: a meta‐analysis of randomized controlled trials. European Journal of Heart Failure 2016;18(7):786‐95. - PubMed
Johnson‐Wimbley 2011
Jordan 2009
Kell 2014
-
- Kell DB, Pretorius E. Serum ferritin is an important inflammatory disease marker as it is mainly a leakage product from damaged cells. Metallomics 2014;6:748‐73. - PubMed
Klein 2017
Klip 2013
-
- Klip IT, Comin‐Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, et al. Iron deficiency in chronic heart failure: an international pooled analysis. American Heart Journal 2013;165:575‐82. - PubMed
Krause 2000
-
- Krause A, Neitz S, Magert HJ, Schulz A, Forssmann WG, Schulz‐Knappe P, et al. LEAP‐1, a novel highly disulphide‐bonded peptide, exhibits antimicrobial activity. FESB Letters 2000;480:147‐50. - PubMed
Lim 2018
-
- Lim J, Miles LF, Litton E. Intravenous iron therapy in patients undergoing cardiovascular surgery: a narrative review. Journal of Cardiothoracic and Vascular Anesthesia 2018;32(3):1439‐51. - PubMed
Litton 2013
Litton 2016
-
- Litton E, Baker S, Erber WN, Farmer S, Ferrier J, French C, et al. Intravenous iron or placebo for anaemia in intensive care: the IRONMAN multicentre randomized blinded trial. Intensive Care Medicine 2016;42:1715‐22. - PubMed
Mascha 2015
-
- Mascha EJ. Alpha, beta, meta: guidelines for assessing power and type I error in meta‐analyses. Anesthesia and Analgesia 2015;121:1430‐3. - PubMed
Melenovsky 2016
-
- Melenovsky V, Petrak J, Mracek T, Benes J, Borlaug BA, Nuskova H, et al. Myocardial iron content and mitochondrial function in human heart failure: a direct tissue analysis. European Journal of Heart Failure 2017;19:522‐30. - PubMed
Miles 2018a
-
- Miles LF, Kunz SA, Na LH, Braat S, Burbury K, Story DA. Postoperative outcome following cardiac surgery in non‐anaemic iron‐replete and iron‐deficient patients ‐ an exploratory study. Anaesthesia 2018;73(4):450‐8. - PubMed
Miles 2019a
-
- Miles LF, Sandhu RN, Grobler AC, Heritier S, Burgess A, Burbury KL, et al. Associations between non‐anaemic iron deficiency and outcomes following surgery for colorectal cancer: an exploratory study of outcomes relevant to prospective observational studies. Anaesthesia and Intensive Care 2019;47(2):152‐9. - PubMed
Miles 2019b
-
- Miles LF, Larsen T, Bailey MJ, Burbury KL, Story DA, Bellomo R. Borderline anaemia and postoperative outcome in women undergoing major abdominal surgery: a retrospective cohort study. Anaesthesia Vol. (in press). - PubMed
Moher 2009
Musallam 2018
-
- Musallam KM, Taher AT. Iron deficiency beyond erythropoiesis: should we be concerned?. Current Medical Research and Opinion 2018;34(1):81‐93. - PubMed
Muñoz 2014
-
- Muñoz M, Gõmez‐Ramírez S, Cuenca J, García‐Erce JA, Iglesias‐Aparicio D, Haman‐Alcober S, et al. Very‐short‐term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion 2014;54:289‐99. - PubMed
Muñoz 2017
-
- Muñoz M, Acheson AG, Auerbach M, Besser M, Habler I, Kehlet H, et al. International consensus statement on the peri‐operative management of anaemia and iron deficiency. Anaesthesia 2017;72:233‐47. - PubMed
National Blood Authority 2012
-
- National Blood Authority. Module 2: Perioperative. Patient Blood Management Guidelines 2012.
Nelson 1994
-
- Nelson M, Bakaliou F, Trivedi A. Iron‐deficiency anaemia and physical performance in adolescent girls from different ethnic backgrounds. British Journal of Nutrition 1994;72:427‐33. - PubMed
Nemeth 2004a
Nemeth 2004b
-
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004;306:2090‐3. - PubMed
Nemeth 2009
Park 2001
-
- Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. Journal of Biological Chemistry 2001;276:7806‐10. - PubMed
Pasricha 2010
-
- Pasricha S, Flecknoe‐Brown SC, Allen KJ, Gibsom PR, McMahon LP, Olynyk JK, et al. Diagnosis and management of iron deficiency anaemia: a clinical update. Medical Journal of Australia 2010;193:525‐32. - PubMed
Pasricha 2013
-
- Pasricha SR, Drakesmith H, Black J, Hipgrave D, Biggs B. Control of iron deficiency anaemia in low and middle income countries. Blood 2013;121:2607‐17. - PubMed
Pratt 2016
Reveiz 2011
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rognoni 2016
Rössler 2019
-
- Rössler J, Schoenrath F, Seifert B, Kaserer A, Spahn GH, Falk V, et al. Iron deficiency is associated with higher mortality in patients undergoing cardiac surgery: a prospective study. British Journal of Anaesthesia Vol. (in press). - PubMed
Schünemann 2019
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Simon 2019
-
- Simon S, Iaonnou A, Deoraj S, Metaxa S, Mendal AK, Missouris CG. Audit of the prevalence and investigation of iron deficiency anaemia in patients with heart failure in hospital practice. Postgraduate Medical Journal Vol. (in press). - PubMed
Stoffel 2017
-
- Stoffel N, Cercamondi CI, Brittenham G, Zeder C, Geurts‐Moespot AJ, Swinkels DW. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice‐daily split dosing in iron‐depleted women: two open‐label, randomised controlled trials. Lancet Haematology 2017;4(11):E524‐33. - PubMed
Suominen 1998
-
- Suominen P, Punnonen K, Rajamäki A, Irjala K. Serum transferrin receptor and transferrin receptor‐ferritin index identify healthy subjects with subclinical iron deficits. Blood 1998;92:2934‐9. - PubMed
von Haehling 2019
-
- Haehling S, Ebner N, Evertz R, Ponikowski P, Anker SD. Iron deficiency in heart failure: an overview. JACC: Heart Failure 2019;7(1):36‐46. - PubMed
Weiss 2005
-
- Weiss G, Goodnough LT. Anaemia of Chronic Disease. New England Journal of Medicine 2005;352:1011‐32. - PubMed
Wetterslev 2009
WHO 2001
-
- World Health Organization, United Nations University, UNICEF. Iron deficiency anemia: assessment, prevention and control. A guide for programme managers. WHO 2001; Vol. www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficienc..., issue (accessed 24 July 2018).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

