Antibacterial Isoquinoline Alkaloids from the Fungus Penicillium Spathulatum Em19
- PMID: 31861067
- PMCID: PMC6943532
- DOI: 10.3390/molecules24244616
Antibacterial Isoquinoline Alkaloids from the Fungus Penicillium Spathulatum Em19
Abstract
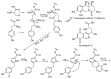
In the search for new microbial antibacterial secondary metabolites, two new compounds (1 and 2) were isolated from culture broths of Penicillium spathulatum Em19. Structure determination by nuclear magnetic resonance and mass spectrometry identified the compounds as 6,7-dihydroxy-5,10-dihydropyrrolo[1,2-b]isoquinoline-3-carboxylic acid (1, spathullin A) and 5,10-dihydropyrrolo[1,2-b]isoquinoline-6,7-diol (2, spathullin B). The two compounds displayed activity against both Gram-negative and -positive bacteria, including Escherichia coli, Acinetobacter baumannii, Enterobacter cloacae, Klebsiella pneumonia, Pseudomonas aeruginosa, and Staphylococcus aureus. Compound 2 was more potent than 1 against all tested pathogens, with minimal inhibitory concentrations down to 1 µg/mL (5 µM) against S. aureus, but 2 was also more cytotoxic than 1 (50% inhibitory concentrations 112 and 11 µM for compounds 1 and 2, respectively, towards Huh7 cells). Based on stable isotope labelling experiments and a literature comparison, the biosynthesis of 1 was suggested to proceed from cysteine, tyrosine and methionine via a non-ribosomal peptides synthase like enzyme complex, whereas compound 2 was formed spontaneously from 1 by decarboxylation. Compound 1 was also easily oxidized to the 1,2-benzoquinone 3. Due to the instability of compound 1 and the toxicity of 2, the compounds are of low interest as possible future antibacterial drugs.
Keywords: Penicillium; antibacterial secondary metabolites; antibiotic resistance; dehydroalanine; isoquinoline alkaloids; secondary metabolism.
Conflict of interest statement
The authors declare no conflict of interest. Medivir AB is a previous funder of the screening program behind this study, and Ultupharma AB is the present funder. Medivir AB and Ultupharma AB approved the publication of the data.
Figures


References
-
- World Health Organization; 2018. [(accessed on 11 September 2019)]. Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2017-2018. Licence: CC BY-NC-SA 3.0 IGO. Available online: https://apps.who.int/iris/bitstream/handle/10665/279656/9789241515061-en....
-
- Drugs@FDA: FDA Approved Drug Products. [(accessed on 24 September 2019)]; Available online: www.fda.gov/drugsatfda.
-
- Srivastava A., Talaue M., Liu S., Degen D., Ebright R.Y., Sineva E., Chakraborty A., Druzhinin S.Y., Chatterjee S., Mukhopadhyay J., et al. New target for inhibition of bacterial RNA polymerase: ‘switch region’. Curr. Opin. Microbiol. 2011;14:532–543. doi: 10.1016/j.mib.2011.07.030. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

