Microtubule-Dependent Trafficking of Alphaherpesviruses in the Nervous System: The Ins and Outs
- PMID: 31861082
- PMCID: PMC6950448
- DOI: 10.3390/v11121165
Microtubule-Dependent Trafficking of Alphaherpesviruses in the Nervous System: The Ins and Outs
Abstract
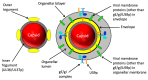
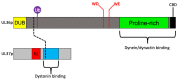
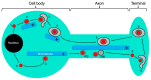
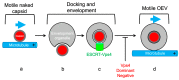
The Alphaherpesvirinae include the neurotropic pathogens herpes simplex virus and varicella zoster virus of humans and pseudorabies virus of swine. These viruses establish lifelong latency in the nuclei of peripheral ganglia, but utilize the peripheral tissues those neurons innervate for productive replication, spread, and transmission. Delivery of virions from replicative pools to the sites of latency requires microtubule-directed retrograde axonal transport from the nerve terminus to the cell body of the sensory neuron. As a corollary, during reactivation newly assembled virions must travel along axonal microtubules in the anterograde direction to return to the nerve terminus and infect peripheral tissues, completing the cycle. Neurotropic alphaherpesviruses can therefore exploit neuronal microtubules and motors for long distance axonal transport, and alternate between periods of sustained plus end- and minus end-directed motion at different stages of their infectious cycle. This review summarizes our current understanding of the molecular details by which this is achieved.
Keywords: anterograde axonal transport; herpes simplex virus; microtubules; motors; neurons; pseudorabies virus; retrograde axonal transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Pellett P.E., Roizman B. Herpesviridae. In: Knipe D.M., Howley P.M., editors. Fields Virology. 6th ed. Volume 2. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2013. pp. 1802–1822.
-
- Roizman B., Knipe D.M., Whitley R.J. Herpes simplex viruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. Volume 2. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2013. pp. 1824–1898.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

