The Spectrum of PAX6 Mutations and Genotype-Phenotype Correlations in the Eye
- PMID: 31861090
- PMCID: PMC6947179
- DOI: 10.3390/genes10121050
The Spectrum of PAX6 Mutations and Genotype-Phenotype Correlations in the Eye
Abstract
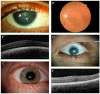
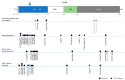
The transcription factor PAX6 is essential in ocular development in vertebrates, being considered the master regulator of the eye. During eye development, it is essential for the correct patterning and formation of the multi-layered optic cup and it is involved in the developing lens and corneal epithelium. In adulthood, it is mostly expressed in cornea, iris, and lens. PAX6 is a dosage-sensitive gene and it is highly regulated by several elements located upstream, downstream, and within the gene. There are more than 500 different mutations described to affect PAX6 and its regulatory regions, the majority of which lead to PAX6 haploinsufficiency, causing several ocular and systemic abnormalities. Aniridia is an autosomal dominant disorder that is marked by the complete or partial absence of the iris, foveal hypoplasia, and nystagmus, and is caused by heterozygous PAX6 mutations. Other ocular abnormalities have also been associated with PAX6 changes, and genotype-phenotype correlations are emerging. This review will cover recent advancements in PAX6 regulation, particularly the role of several enhancers that are known to regulate PAX6 during eye development and disease. We will also present an updated overview of the mutation spectrum, where an increasing number of mutations in the non-coding regions have been reported. Novel genotype-phenotype correlations will also be discussed.
Keywords: PAX6; aniridia; enhancers; genotype-phenotype correlations; haploinsufficiency; microphthalmia; non-coding variants; paired domain; premature termination codon; regulatory regions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ton C.C., Hirvonen H., Miwa H., Weil M.M., Monaghan P., Jordan T., van Heyningen V., Hastie N.D., Meijers-Heijboer H., Drechsler M., et al. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell. 1991;67:1059–1074. doi: 10.1016/0092-8674(91)90284-6. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

