Novel Variants of Angiotensin Converting Enzyme-2 of Shorter Molecular Size to Target the Kidney Renin Angiotensin System
- PMID: 31861139
- PMCID: PMC6995632
- DOI: 10.3390/biom9120886
Novel Variants of Angiotensin Converting Enzyme-2 of Shorter Molecular Size to Target the Kidney Renin Angiotensin System
Abstract
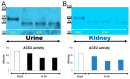
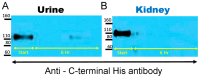
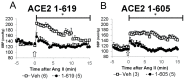
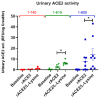
ACE2 is a monocarboxypeptidase which generates Angiotensin (1-7) from Angiotensin II (1-8). Attempts to target the kidney Renin Angiotensin System using native ACE2 to treat kidney disease are hampered by its large molecular size, 100 kDa, which precludes its glomerular filtration and subsequent tubular uptake. Here, we show that both urine and kidney lysates are capable of digesting native ACE2 into shorter proteins of ~60-75 kDa and then demonstrate that they are enzymatically very active. We then truncated the native ACE2 by design from the C-terminus to generate two short recombinant (r)ACE2 variants (1-605 and 1-619AA). These two truncates have a molecular size of ~70 kDa, as expected from the amino acid sequence and as shown by Western blot. ACE2 enzyme activity, measured using a specific substrate, was higher than that of the native rACE2 (1-740 AA). When infused to mice with genetic ACE2 deficiency, a single i.v. injection of 1-619 resulted in detectable ACE2 activity in urine, whereas infusion of the native ACE2 did not. Moreover, ACE2 activity was recovered in harvested kidneys from ACE2-deficient mice infused with 1-619, but not in controls (23.1 ± 4.3 RFU/µg creatinine/h and 1.96 ± 0.73 RFU/µg protein/hr, respectively). In addition, the kidneys of ACE2-null mice infused with 1-619 studied ex vivo formed more Ang (1-7) from exogenous Ang II than those infused with vehicle (AUC 8555 ± 1933 vs. 3439 ± 753 ng/mL, respectively, p < 0.05) further demonstrating the functional effect of increasing kidney ACE2 activity after the infusion of our short ACE2 1-619 variant. We conclude that our novel short recombinant ACE2 variants undergo glomerular filtration, which is associated with kidney uptake of enzymatically active proteins that can enhance the formation of Ang (1-7) from Ang II. These small ACE2 variants may offer a potentially useful approach to target kidney RAS overactivity to combat kidney injury.
Keywords: ACE2; Acute Kidney Injury; Angiotensin (1-7); Angiotensin II; Renin Angiotensin System.
Conflict of interest statement
D. Batlle and J. Wysocki: co-inventors Patent: ‘Active Low Molecular Weight Variants of Angiotensin Converting Enzyme 2′; D. Batlle: Founder of ‘Angiotensin Therapeutics’.
Figures






References
-
- Santos R.A., Simoes e Silva A.C., Maric C., Silva D.M., Machado R.P., de Buhr I., Heringer-Walther S., Pinheiro S.V., Lopes M.T., Bader M., et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc. Natl. Acad. Sci. USA. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. - DOI - PMC - PubMed
-
- Ferrario C.M., Jessup J., Gallagher P.E., Averill D.B., Brosnihan K.B., Ann Tallant E., Smith R.D., Chappell M.C. Effects of renin-angiotensin system blockade on renal angiotensin-(1-7) forming enzymes and receptors. Kidney Int. 2005;68:2189–2196. doi: 10.1111/j.1523-1755.2005.00675.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

