A Review of Key Biological and Molecular Events Underpinning Transformation of Melanocytes to Primary and Metastatic Melanoma
- PMID: 31861163
- PMCID: PMC6966527
- DOI: 10.3390/cancers11122041
A Review of Key Biological and Molecular Events Underpinning Transformation of Melanocytes to Primary and Metastatic Melanoma
Abstract
Melanoma is a major public health concern that is responsible for significant morbidity and mortality, particularly in countries such as New Zealand and Australia where it is the commonest cause of cancer death in young adults. Until recently, there were no effective drug therapies for patients with advanced melanoma however significant advances in our understanding of the biological and molecular basis of melanoma in recent decades have led to the development of revolutionary treatments, including targeted molecular therapy and immunotherapy. This review summarizes our current understanding of the key events in the pathway of melanomagenesis and discusses the role of genomic analysis as a potential tool for improved diagnostic evaluation, prognostication and treatment strategies. Ultimately, it is hoped that a continued deeper understanding of the mechanisms of melanomagenesis will lead to the development of even more effective treatments that continue to provide better outcomes for patients with melanoma.
Keywords: diagnosis; melanoma; melanomagenesis; metastasis; pathology; progression; treatment.
Conflict of interest statement
R.A.S. has received fees for professional services from Merck Sharp & Dohme, GlaxoSmithKline Australia, Bristol-Myers Squibb, Dermpedia, Novartis Pharmaceuticals Australia Pty Ltd, Myriad, NeraCare and Amgen.
Figures

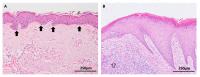
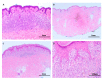
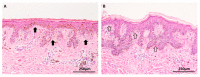
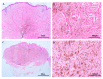


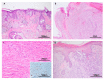
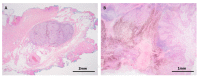
References
-
- Melanoma of the Skin. Australian Institute of Health and Welfare; Canberra, Australia: 2016.
Publication types
LinkOut - more resources
Full Text Sources

