Neurologic Injury and Brain Growth in the Setting of Long-Gap Esophageal Atresia Perioperative Critical Care: A Pilot Study
- PMID: 31861169
- PMCID: PMC6955668
- DOI: 10.3390/brainsci9120383
Neurologic Injury and Brain Growth in the Setting of Long-Gap Esophageal Atresia Perioperative Critical Care: A Pilot Study
Abstract
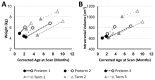
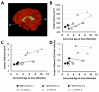
We previously showed that infants born with long-gap esophageal atresia (LGEA) demonstrate clinically significant brain MRI findings following repair with the Foker process. The current pilot study sought to identify any pre-existing (PRE-Foker process) signs of brain injury and to characterize brain and corpus callosum (CC) growth. Preterm and full-term infants (n = 3/group) underwent non-sedated brain MRI twice: before (PRE-Foker scan) and after (POST-Foker scan) completion of perioperative care. A neuroradiologist reported on qualitative brain findings. The research team quantified intracranial space, brain, cerebrospinal fluid (CSF), and CC volumes. We report novel qualitative brain findings in preterm and full-term infants born with LGEA before undergoing Foker process. Patients had a unique hospital course, as assessed by secondary clinical end-point measures. Despite increased total body weight and absolute intracranial and brain volumes (cm3) between scans, normalized brain volume was decreased in 5/6 patients, implying delayed brain growth. This was accompanied by both an absolute and relative CSF volume increase. In addition to qualitative findings of CC abnormalities in 3/6 infants, normative CC size (% brain volume) was consistently smaller in all infants, suggesting delayed or abnormal CC maturation. A future larger study group is warranted to determine the impact on the neurodevelopmental outcomes of infants born with LGEA.
Keywords: LGEA; MRI; infant; midazolam; morphine; neuroimaging; opioids; term; tolerance; weaning.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

