Development of a Resveratrol Nanosuspension Using the Antisolvent Precipitation Method without Solvent Removal, Based on a Quality by Design (QbD) Approach
- PMID: 31861173
- PMCID: PMC6955680
- DOI: 10.3390/pharmaceutics11120688
Development of a Resveratrol Nanosuspension Using the Antisolvent Precipitation Method without Solvent Removal, Based on a Quality by Design (QbD) Approach
Abstract
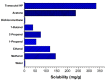
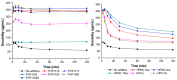
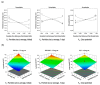
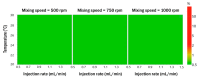
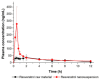
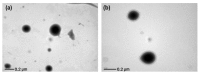
The purpose of this study was to develop a resveratrol nanosuspension with enhanced oral bioavailability, based on an understanding of the formulation and process parameters of nanosuspensions and using a quality by design (QbD) approach. Particularly, the antisolvent method, which requires no solvent removal and no heating, is newly applied to prepare resveratrol nanosuspension. To ensure the quality of the resveratrol nanosuspensions, a quality target product profile (QTPP) was defined. The particle size (z-average, d90), zeta potential, and drug content parameters affecting the QTPP were selected as critical quality attributes (CQAs). The optimum composition obtained using a 3-factor, 3-level Box-Behnken design was as follows: polyvinylpyrrolidone vinyl acetate (10 mg/mL), polyvinylpyrrolidone K12 (5 mg/mL), sodium lauryl sulfate (1 mg/mL), and diethylene glycol monoethyl ether (DEGEE, 5% v/v) at a resveratrol concentration of 5 mg/mL. The initial particle size (z-average) was 46.3 nm and the zeta potential was -38.02 mV. The robustness of the antisolvent process using the optimized composition conditions was ensured by a full factorial design. The dissolution rate of the optimized resveratrol nanosuspension was significantly greater than that of the resveratrol raw material. An in vivo pharmacokinetic study in rats showed that the area under the plasma concentration versus time curve (AUC0-12h) and the maximum plasma concentration (Cmax) respectively, than those of the resveratrol raw material. Therefore, the prepara values of the resveratrol nanosuspension were approximately 1.6- and 5.7-fold higher,tion of a resveratrol nanosuspension using the QbD approach may be an effective strategy for the development of a new dosage form of resveratrol, with enhanced oral bioavailability.
Keywords: bioavailability; dissolution; nanosuspension; optimization; quality by design; resveratrol.
Conflict of interest statement
The authors declare no conflict of interest. Jeong-Soo Kim is the employee of the Dong-A ST Co. Ltd. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources

