Cardiac Extracellular Vesicles (EVs) Released in the Presence or Absence of Inflammatory Cues Support Angiogenesis in Different Manners
- PMID: 31861211
- PMCID: PMC6940836
- DOI: 10.3390/ijms20246363
Cardiac Extracellular Vesicles (EVs) Released in the Presence or Absence of Inflammatory Cues Support Angiogenesis in Different Manners
Abstract
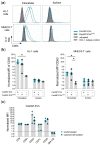
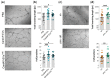
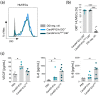
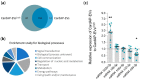
Cells release extracellular vesicles (EVs) to communicate in a paracrine manner with other cells, and thereby influence processes, such as angiogenesis. The conditioned medium of human cardiac-derived adherent proliferating (CardAP) cells was recently shown to enhance angiogenesis. To elucidate whether their released EVs are involved, we isolated them by differential centrifugation from the conditioned medium derived either in the presence or absence of a pro-inflammatory cytokine cocktail. Murine recipient cells internalized CardAP-EVs as determined by an intracellular detection of human proteins, such as CD63, by a novel flow cytometry method for studying EV-cell interaction. Moreover, endothelial cells treated for 24 h with either unstimulated or cytokine stimulated CardAP-EVs exhibited a higher tube formation capability on Matrigel. Interestingly, unstimulated CardAP-EVs caused endothelial cells to release significantly more vascular endothelial growth factor and interleukin (IL)-6, while cytokine stimulated CardAP-EVs significantly enhanced the release of IL-6 and IL-8. By nCounter® miRNA expression assay (NanoString Technologies) we identified microRNA 302d-3p to be enhanced in unstimulated CardAP-EVs compared to their cytokine stimulated counterparts, which was verified by quantitative polymerase chain reaction. This study demonstrates that both CardAP-EVs are pro-angiogenic by inducing different factors from endothelial cells. This would allow to select potent targets for a safe and efficient therapeutic application.
Keywords: VEGF; angiogenesis; cardiac therapy; extracellular vesicles; intracellular uptake; regeneration.
Conflict of interest statement
M. Sittinger is a shareholder of CellServe GmbH (Berlin, Germany) and BioRetis GmbH (Berlin, Germany). CellServe owns a license for CardAP cells. M. Haag and M. Sittinger are investors of patent EP000002129774A1 “Cells for heart treatment”. The remaining authors report no competing financial interests.
Figures

Similar articles
-
Extracellular vesicles from regenerative human cardiac cells act as potent immune modulators by priming monocytes.J Nanobiotechnology. 2019 May 27;17(1):72. doi: 10.1186/s12951-019-0504-0. J Nanobiotechnology. 2019. PMID: 31133024 Free PMC article.
-
Extracellular Vesicles Derived from Endothelial Progenitor Cells Protect Human Glomerular Endothelial Cells and Podocytes from Complement- and Cytokine-Mediated Injury.Cells. 2021 Jul 2;10(7):1675. doi: 10.3390/cells10071675. Cells. 2021. PMID: 34359843 Free PMC article.
-
MicroRNA-containing extracellular vesicles released from endothelial colony-forming cells modulate angiogenesis during ischaemic retinopathy.J Cell Mol Med. 2017 Dec;21(12):3405-3419. doi: 10.1111/jcmm.13251. Epub 2017 Jun 20. J Cell Mol Med. 2017. PMID: 28631889 Free PMC article.
-
Extracellular Vesicles as Mediators of Cellular Crosstalk Between Immune System and Kidney Graft.Front Immunol. 2020 Feb 27;11:74. doi: 10.3389/fimmu.2020.00074. eCollection 2020. Front Immunol. 2020. PMID: 32180768 Free PMC article. Review.
-
The Emerging Role of Pericyte-Derived Extracellular Vesicles in Vascular and Neurological Health.Cells. 2022 Oct 2;11(19):3108. doi: 10.3390/cells11193108. Cells. 2022. PMID: 36231071 Free PMC article. Review.
Cited by
-
Extracellular Vesicles and Cell-Cell Communication: New Insights and New Therapeutic Strategies Not Only in Oncology.Int J Mol Sci. 2020 Jun 18;21(12):4331. doi: 10.3390/ijms21124331. Int J Mol Sci. 2020. PMID: 32570703 Free PMC article.
-
The tumor therapeutic potential of long non-coding RNA delivery and targeting.Acta Pharm Sin B. 2023 Apr;13(4):1371-1382. doi: 10.1016/j.apsb.2022.12.005. Epub 2022 Dec 13. Acta Pharm Sin B. 2023. PMID: 37139413 Free PMC article. Review.
-
Stem Cell Studies in Cardiovascular Biology and Medicine: A Possible Key Role of Macrophages.Biology (Basel). 2022 Jan 12;11(1):122. doi: 10.3390/biology11010122. Biology (Basel). 2022. PMID: 35053119 Free PMC article. Review.
-
Extracellular Vesicles' Role in Angiogenesis and Altering Angiogenic Signaling.Med Sci (Basel). 2024 Jan 3;12(1):4. doi: 10.3390/medsci12010004. Med Sci (Basel). 2024. PMID: 38249080 Free PMC article. Review.
References
-
- Collino F., Bruno S., Deregibus M.C., Tetta C., Camussi G. MicroRNAs and Mesenchymal Stem Cells. Vitam. Horm. 2011;87:291–320. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

