Advanced Glycation End Products (AGEs) May Be a Striking Link Between Modern Diet and Health
- PMID: 31861217
- PMCID: PMC6995512
- DOI: 10.3390/biom9120888
Advanced Glycation End Products (AGEs) May Be a Striking Link Between Modern Diet and Health
Abstract
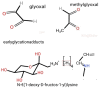
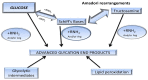
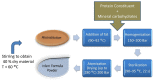
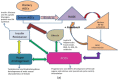
The Maillard reaction is a simple but ubiquitous reaction that occurs both in vivo and ex vivo during the cooking or processing of foods under high-temperature conditions, such as baking, frying, or grilling. Glycation of proteins is a post-translational modification that forms temporary adducts, which, on further crosslinking and rearrangement, form permanent residues known as advanced glycation end products (AGEs). Cooking at high temperature results in various food products having high levels of AGEs. This review underlines the basis of AGE formation and their corresponding deleterious effects on the body. Glycated Maillard products have a direct association with the pathophysiology of some metabolic diseases, such as diabetes mellitus type 2 (DM2), acute renal failure (ARF), Alzheimer's disease, dental health, allergies, and polycystic ovary syndrome (PCOS). The most glycated and structurally abundant protein is collagen, which acts as a marker for diabetes and aging, where decreased levels indicate reduced skin elasticity. In diabetes, high levels of AGEs are associated with carotid thickening, ischemic heart disease, uremic cardiomyopathy, and kidney failure. AGEs also mimic hormones or regulate/modify their receptor mechanisms at the DNA level. In women, a high AGE diet directly correlates with high levels of androgens, anti-Müllerian hormone, insulin, and androstenedione, promoting ovarian dysfunction and/or infertility. Vitamin D3 is well-associated with the pathogenesis of PCOS and modulates steroidogenesis. It also exhibits a protective mechanism against the harmful effects of AGEs. This review elucidates and summarizes the processing of infant formula milk and the associated health hazards. Formulated according to the nutritional requirements of the newborn as a substitute for mother's milk, formula milk is a rich source of primary adducts, such as carboxy-methyl lysine, which render an infant prone to inflammation, dementia, food allergies, and other diseases. We therefore recommend that understanding this post-translational modification is the key to unlocking the mechanisms and physiology of various metabolic syndromes.
Keywords: Alzheimer’s disease; Maillard reaction; advanced glycation end products; diabetes; infant formula; polycystic ovarian syndrome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Do Advanced Glycation End Products (AGEs) Contribute to the Comorbidities of Polycystic Ovary Syndrome (PCOS)?Curr Pharm Des. 2016;22(36):5558-5571. doi: 10.2174/1381612822666160714094404. Curr Pharm Des. 2016. PMID: 27412301 Review.
-
Crosstalk between advanced glycation end products and vitamin D: A compelling paradigm for the treatment of ovarian dysfunction in PCOS.Mol Cell Endocrinol. 2019 Jan 5;479:20-26. doi: 10.1016/j.mce.2018.08.010. Epub 2018 Aug 28. Mol Cell Endocrinol. 2019. PMID: 30170183 Review.
-
Contribution of Advanced Glycation End Products to PCOS Key Elements: A Narrative Review.Nutrients. 2022 Aug 30;14(17):3578. doi: 10.3390/nu14173578. Nutrients. 2022. PMID: 36079834 Free PMC article. Review.
-
Advanced glycation endproducts in food and their effects on health.Food Chem Toxicol. 2013 Oct;60:10-37. doi: 10.1016/j.fct.2013.06.052. Epub 2013 Jul 16. Food Chem Toxicol. 2013. PMID: 23867544 Review.
-
Advanced glycation end product signaling and metabolic complications: Dietary approach.World J Diabetes. 2023 Jul 15;14(7):995-1012. doi: 10.4239/wjd.v14.i7.995. World J Diabetes. 2023. PMID: 37547584 Free PMC article. Review.
Cited by
-
Research Progress in Skin Aging, Metabolism, and Related Products.Int J Mol Sci. 2023 Nov 3;24(21):15930. doi: 10.3390/ijms242115930. Int J Mol Sci. 2023. PMID: 37958920 Free PMC article. Review.
-
Skin Aging and the Upcoming Role of Ferroptosis in Geroscience.Int J Mol Sci. 2024 Jul 28;25(15):8238. doi: 10.3390/ijms25158238. Int J Mol Sci. 2024. PMID: 39125810 Free PMC article. Review.
-
Functional Activities and Mechanisms of Aronia melanocarpa in Our Health.Curr Issues Mol Biol. 2024 Jul 26;46(8):8071-8087. doi: 10.3390/cimb46080477. Curr Issues Mol Biol. 2024. PMID: 39194694 Free PMC article. Review.
-
The Potential Use of Vitamin D3 and Phytochemicals for Their Anti-Ageing Effects.Int J Mol Sci. 2024 Feb 9;25(4):2125. doi: 10.3390/ijms25042125. Int J Mol Sci. 2024. PMID: 38396804 Free PMC article. Review.
-
Selective loss of kisspeptin signaling in oocytes causes progressive premature ovulatory failure.Hum Reprod. 2022 Apr 1;37(4):806-821. doi: 10.1093/humrep/deab287. Hum Reprod. 2022. PMID: 35037941 Free PMC article.
References
-
- Steinhart H. The Maillard Reaction. Chemistry, Biochemistry and Implications. By Harry Nursten. Angew. Int. Ed. 2005;44:7503–7504. doi: 10.1002/anie.200585332. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

