The Phenotype and Secretory Activity of Adipose-Derived Mesenchymal Stem Cells (ASCs) of Patients with Rheumatic Diseases
- PMID: 31861245
- PMCID: PMC6952982
- DOI: 10.3390/cells8121659
The Phenotype and Secretory Activity of Adipose-Derived Mesenchymal Stem Cells (ASCs) of Patients with Rheumatic Diseases
Abstract
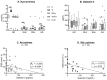
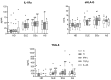
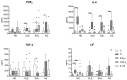
Mesenchymal stem/stromal cells (MSCs) have immunosuppressive and regenerative properties. Adipose tissue is an alternative source of MSCs, named adipose-derived mesenchymal stem cells (ASCs). Because the biology of ASCs in rheumatic diseases (RD) is poorly understood, we performed a basic characterization of RD/ASCs. The phenotype and expression of adhesion molecules (intracellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1) on commercially available healthy donors (HD), ASC lines (n = 5) and on ASCs isolated from patients with systemic lupus erythematosus (SLE, n = 16), systemic sclerosis (SSc, n = 17) and ankylosing spondylitis (AS, n = 16) were analyzed by flow cytometry. The secretion of immunomodulatory factors by untreated and cytokine-treated ASCs was measured by ELISA. RD/ASCs have reduced basal levels of CD90 and ICAM-1 expression, correlated with interleukin (IL)-6 and transforming growth factor (TGF)-β1 release, respectively. Compared with HD/ASCs, untreated and tumour necrosis factor (TNF) + interferon (IFN)-γ (TI)-treated RD/ASCs produced similar amounts of prostaglandin E2 (PGE2), IL-6, leukemia inhibiting factor (LIF), and TGF-β1, more IL-1Ra, soluble human leukocyte antigen G (sHLA-G) and tumor necrosis factor-inducible gene (TSG)-6, but less kynurenines and galectin-3. Basal secretion of galectin-3 was inversely correlated with the patient's erythrocyte sedimentation rate (ESR) value. IFN-α and IL-23 slightly raised galectin-3 release from SLE/ASCs and AS/ASCs, respectively. TGF-β1 up-regulated PGE2 secretion by SSc/ASCs. In conclusion, RD/ASCs are characterized by low basal levels of CD90 and ICAM-1 expression, upregulated secretion of IL-1Ra, TSG-6 and sHLA-G, but impaired release of kynurenines and galectin-3. These abnormalities may modify biological activities of RD/ASCs.
Keywords: adipose-derived mesenchymal stem cells; ankylosing spondylitis; phenotype; secretory potential; systemic lupus erythematosus; systemic sclerosis.
Conflict of interest statement
The authors declare that they have no competing interests. The funder (National Science Centre) had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Modulation of T-Cell Activation Markers Expression by the Adipose Tissue-Derived Mesenchymal Stem Cells of Patients with Rheumatic Diseases.Cell Transplant. 2020 Jan-Dec;29:963689720945682. doi: 10.1177/0963689720945682. Cell Transplant. 2020. PMID: 32878464 Free PMC article.
-
Impact and Possible Mechanism(s) of Adipose Tissue-Derived Mesenchymal Stem Cells on T-Cell Proliferation in Patients With Rheumatic Disease.Front Physiol. 2022 Jan 13;12:749481. doi: 10.3389/fphys.2021.749481. eCollection 2021. Front Physiol. 2022. PMID: 35095547 Free PMC article.
-
Direct anti-proliferative effect of adipose-derived mesenchymal stem cells of ankylosing spondylitis patients on allogenic CD4+ cells.Reumatologia. 2021;59(1):12-22. doi: 10.5114/reum.2021.103940. Epub 2021 Feb 28. Reumatologia. 2021. PMID: 33707791 Free PMC article.
-
Mesenchymal stromal cells for bone sarcoma treatment: Roadmap to clinical practice.J Bone Oncol. 2019 Mar 19;16:100231. doi: 10.1016/j.jbo.2019.100231. eCollection 2019 Jun. J Bone Oncol. 2019. PMID: 30956944 Free PMC article. Review.
-
Responses of adipose-derived stem cells during hypoxia: enhanced skin-regenerative potential.Expert Opin Biol Ther. 2009 Dec;9(12):1499-508. doi: 10.1517/14712590903307362. Expert Opin Biol Ther. 2009. PMID: 19780713 Review.
Cited by
-
Inhibition of Allogeneic and Autologous T Cell Proliferation by Adipose-Derived Mesenchymal Stem Cells of Ankylosing Spondylitis Patients.Stem Cells Int. 2021 Mar 12;2021:6637328. doi: 10.1155/2021/6637328. eCollection 2021. Stem Cells Int. 2021. PMID: 33777148 Free PMC article.
-
Clinical applications of mesenchymal stromal cell-based therapies for pulmonary diseases: An Update and Concise Review.Int J Med Sci. 2021 Jun 1;18(13):2849-2870. doi: 10.7150/ijms.59218. eCollection 2021. Int J Med Sci. 2021. PMID: 34220313 Free PMC article.
-
Basic Properties of Adipose-Derived Mesenchymal Stem Cells of Rheumatoid Arthritis and Osteoarthritis Patients.Pharmaceutics. 2023 Mar 20;15(3):1003. doi: 10.3390/pharmaceutics15031003. Pharmaceutics. 2023. PMID: 36986863 Free PMC article.
-
Adipose-Derived Stem Cells From Patients With Ulcerative Colitis Exhibit Impaired Immunosuppressive Function.Front Cell Dev Biol. 2022 Feb 18;10:822772. doi: 10.3389/fcell.2022.822772. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35252190 Free PMC article.
-
Factors Defining Human Adipose Stem/Stromal Cell Immunomodulation in Vitro.Stem Cell Rev Rep. 2024 Jan;20(1):175-205. doi: 10.1007/s12015-023-10654-7. Epub 2023 Nov 14. Stem Cell Rev Rep. 2024. PMID: 37962697 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

