The Combination of MoS2/WO3 and Its Adsorption Properties of Methylene Blue at Low Temperatures
- PMID: 31861262
- PMCID: PMC6982728
- DOI: 10.3390/molecules25010002
The Combination of MoS2/WO3 and Its Adsorption Properties of Methylene Blue at Low Temperatures
Abstract
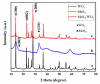
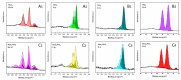
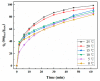
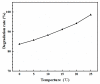
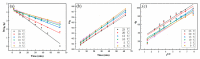
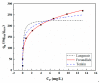
It was found previously that neither monomer MoS2 nor WO3 is an ideal material for the adsorption of organic dyes, while MoS2/WO3 composites synthesized by a two-step hydrothermal method have outstanding adsorption effects. In this work, the chemical state of each element was found to be changed after combination by X-ray photoelectron spectroscopy analysis, which lead to their differences in adsorption performance. Moreover, the adsorption test of methylene blue on MoS2/WO3 composites was carried out under a series of temperatures, showing that the prepared composites also had appreciable adsorption rates at lower temperatures. The adsorption process could be well described by the Freundlich isothermal model and the pseudo-second order model. In addition, the particle-internal diffusion model simulation revealed that the internal diffusion of the particles played an important role in the whole adsorption process.
Keywords: MoS2/WO3; adsorption; combination; methylene blue.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Defect-engineered dual Z-scheme core-shell MoS2/WO3-x/AgBiS2 for antibiotic and dyes degradation in photo and night catalysis: Mechanism and pathways.Environ Pollut. 2024 Sep 1;356:124375. doi: 10.1016/j.envpol.2024.124375. Epub 2024 Jun 14. Environ Pollut. 2024. PMID: 38880327
-
Adsorption kinetics and Box-Behnken design optimization for organic dyes on tungsten oxide.Environ Technol. 2022 Jul;43(17):2620-2636. doi: 10.1080/09593330.2021.1892199. Epub 2021 Mar 2. Environ Technol. 2022. PMID: 33594941
-
Synthesis of tungsten oxide/ amino-functionalized sugarcane bagasse derived-carbon quantum dots (WO3/N-CQDs) composites for methylene blue removal.Chemosphere. 2021 Aug;277:130300. doi: 10.1016/j.chemosphere.2021.130300. Epub 2021 Mar 18. Chemosphere. 2021. PMID: 33774232
-
Mutual effects behind the simultaneous removal of toxic metals and cationic dyes by interlayer-expanded MoS2 nanosheets.Environ Sci Pollut Res Int. 2019 Oct;26(30):31344-31353. doi: 10.1007/s11356-019-06277-6. Epub 2019 Aug 30. Environ Sci Pollut Res Int. 2019. PMID: 31471849
-
Nigella sativa seed based nanohybrid composite-Fe2O3-SnO2/BC: A novel material for enhanced adsorptive removal of methylene blue from water.Environ Res. 2019 Nov;178:108667. doi: 10.1016/j.envres.2019.108667. Epub 2019 Aug 16. Environ Res. 2019. PMID: 31454728
Cited by
-
Ex Situ Synthesis and Characterizations of MoS2/WO3 Heterostructures for Efficient Photocatalytic Degradation of RhB.Nanomaterials (Basel). 2022 Aug 28;12(17):2974. doi: 10.3390/nano12172974. Nanomaterials (Basel). 2022. PMID: 36080010 Free PMC article.
-
Temperature-Based Selective Detection of Hydrogen Sulfide and Ethanol with MoS2/WO3 Composite.ACS Omega. 2022 Feb 7;7(7):6075-6085. doi: 10.1021/acsomega.1c06471. eCollection 2022 Feb 22. ACS Omega. 2022. PMID: 35224369 Free PMC article.
References
-
- Xiao X., Sun Y., Sun W., Shen H., Zheng H., Xu Y., Zhao J., Wu H., Liu C. Advanced treatment of actual textile dye wastewater by Fenton-flocculation process. Can. J. Chem. Eng. 2017;7:1245–1252. doi: 10.1002/cjce.22752. - DOI
-
- Malik P.K., Saha S.K. Oxidation of direct dyes with hydrogen peroxide using ferrous ion as catalyst. Sep. Purif. Technol. 2003;3:241–250. doi: 10.1016/S1383-5866(02)00200-9. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

