Alpha Glucosidase Inhibitory Activities of Plants with Focus on Common Vegetables
- PMID: 31861279
- PMCID: PMC7020213
- DOI: 10.3390/plants9010002
Alpha Glucosidase Inhibitory Activities of Plants with Focus on Common Vegetables
Abstract
Type-2 diabetes mellitus is one of the most prevalent metabolic diseases in the world, and is characterized by hyperglycemia (i.e., high levels of glucose in the blood). Alpha-glucosidases are enzymes in the digestive tract that hydrolyze carbohydrates into glucose. One strategy that has been developed to treat type-2 diabetes is inhibition of the activity of alpha-glucosidases using synthetic drugs. However, these inhibitors are usually associated with gastrointestinal side effects. Therefore, the development of inhibitors from natural products offers an alternative option for the control of hyperglycemia. In recent years, various studies have been conducted to identify alpha-glucosidases inhibitors from natural sources such as plants, and many candidates have transpired to be secondary metabolites including alkaloids, flavonoids, phenols, and terpenoids. In this review, we focus on the alpha-glucosidases inhibitors found in common vegetable crops and the major classes of phytochemicals responsible for the inhibitory activity, and also as potential/natural drug candidates for the treatment of type-2 diabetes mellitus. In addition, possible breeding strategies for production of improved vegetable crops with higher content of the inhibitors are also described.
Keywords: alpha-glucosidase; alpha-glucosidase inhibitor; breeding; diabetes; secondary metabolites; vegetables.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this article.
Figures
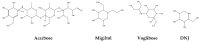
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

