Thrombolytic Potential of Novel Thiol-Dependent Fibrinolytic Protease from Bacillus cereus RSA1
- PMID: 31861284
- PMCID: PMC7022875
- DOI: 10.3390/biom10010003
Thrombolytic Potential of Novel Thiol-Dependent Fibrinolytic Protease from Bacillus cereus RSA1
Abstract
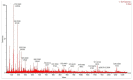
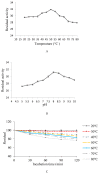
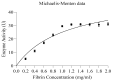
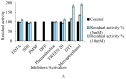
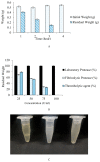
The present study demonstrates the production and thrombolytic potential of a novel thermostable thiol-dependent fibrinolytic protease by Bacillus cereus RSA1. Statistical optimization of different parameters was accomplished with Plackett-Burman design and validated further by central composite design with 30.75 U/mL protease production. Precipitation and chromatographic approaches resulted in 33.11% recovery with 2.32-fold purification. The molecular weight of fibrinolytic protease was 40 KDa and it exhibited a broad temperature and pH stability range of 20-80 °C and pH 5-10 with utmost activity at 50 °C and pH 8, respectively. The protease retained its fibrinolytic activity in organic solvents and enhanced the activity in solutions with divalent cations (Mn2+, Zn2+, and Cu2+). The enzyme kinetics revealed Km and Vmax values of 1.093 mg/mL and 52.39 µg/mL/min, respectively, indicating higher affinity of fibrinolytic activity towards fibrin. Also, complete inhibition of fibrinolytic activity with DFP and a 2-fold increase with DTT and β-mercaptoethanol indicates its thiol-dependent serine protease nature. MALDI-TOF analysis showed 56% amino acid sequence homology with Subtilisin NAT OS = Bacillus subtilis subsp. natto. The fibrinolysis activity was compared with a commercial thrombolytic agent for its therapeutic applicability, and fibrinolytic protease was found highly significant with absolute blood clot dissolution within 4 h in in vitro conditions. The isolated fibrinolytic protease of Bacillus cereus RSA1 is novel and different from other known fibrinolytic proteases with high stability and efficacy, which might have wide medicinal and industrial application as a thrombolytic agent and in blood stain removal, respectively.
Keywords: Bacillus cereus RSA1; fibrinolytic protease; thiol-dependent; thrombolytic potential.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Purification, biochemical, and thermal properties of fibrinolytic enzyme secreted by Bacillus cereus SRM-001.Prep Biochem Biotechnol. 2018 Jan 2;48(1):34-42. doi: 10.1080/10826068.2017.1387560. Epub 2018 Jan 3. Prep Biochem Biotechnol. 2018. PMID: 29106326
-
Fibrinolytic protease from Bacillus cereus S46: Purification, characterization, and evaluation of its in vitro thrombolytic potential.J Basic Microbiol. 2020 Aug;60(8):661-668. doi: 10.1002/jobm.202000148. Epub 2020 Jun 9. J Basic Microbiol. 2020. PMID: 32515847
-
Statistical optimization of fibrinolytic enzyme production using agroresidues by Bacillus cereus IND1 and its thrombolytic activity in vitro.Biomed Res Int. 2014;2014:725064. doi: 10.1155/2014/725064. Epub 2014 Jun 9. Biomed Res Int. 2014. PMID: 25003130 Free PMC article.
-
Research Progress on Douchi Fibrinolytic Enzyme.Protein J. 2025 Apr;44(2):162-174. doi: 10.1007/s10930-025-10262-z. Epub 2025 Mar 13. Protein J. 2025. PMID: 40082379 Review.
-
Marine Microbial Fibrinolytic Enzymes: An Overview of Source, Production, Biochemical Properties and Thrombolytic Activity.Mar Drugs. 2022 Jan 2;20(1):46. doi: 10.3390/md20010046. Mar Drugs. 2022. PMID: 35049901 Free PMC article. Review.
Cited by
-
Role of Fibrinolytic Enzymes in Anti-Thrombosis Therapy.Front Mol Biosci. 2021 May 28;8:680397. doi: 10.3389/fmolb.2021.680397. eCollection 2021. Front Mol Biosci. 2021. PMID: 34124160 Free PMC article. Review.
-
Computational-approach understanding the structure-function prophecy of Fibrinolytic Protease RFEA1 from Bacillus cereus RSA1.PeerJ. 2021 Jun 4;9:e11570. doi: 10.7717/peerj.11570. eCollection 2021. PeerJ. 2021. PMID: 34141495 Free PMC article.
-
Microbial Fibrinolytic Enzymes as Anti-Thrombotics: Production, Characterisation and Prodigious Biopharmaceutical Applications.Pharmaceutics. 2021 Nov 5;13(11):1880. doi: 10.3390/pharmaceutics13111880. Pharmaceutics. 2021. PMID: 34834294 Free PMC article. Review.
-
Emergence of MDR Enterobacter hormaechei RSM5 in Pharma Effluent and its Implications in β-lactam Antibiotic Removal from Effluent.Curr Microbiol. 2025 Feb 7;82(3):127. doi: 10.1007/s00284-025-04103-6. Curr Microbiol. 2025. PMID: 39920455
-
An Insight into Efflux-Mediated Arsenic Resistance and Biotransformation Potential of Enterobacter Cloacae RSC3 from Arsenic Polluted Area.Indian J Microbiol. 2022 Sep;62(3):456-467. doi: 10.1007/s12088-022-01028-7. Epub 2022 Jun 1. Indian J Microbiol. 2022. PMID: 35974925 Free PMC article.
References
-
- World Heart Federation The World’s Most Common Cause of Death, Cardiovascular Diseases (CVDs), Global Facts and Figures. [(accessed on 18 December 2019)]; Available online: https://www.world-heart-federation.org/wp-content/uploads/2017/05/WCC201....
-
- Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., Chiuve S.E., Cushman M., Delling F.N., Deo R., et al. Heart disease and stroke statistics-2018 update: A report from the American Heart Association. Circulation. 2018;137:67. doi: 10.1161/CIR.0000000000000558. - DOI - PubMed
-
- Jeong Y.K., Kim J.H., Gal S.W., Kim J.E., Park S.S., Chung K.T., Kim Y.H., Kim B.W., Joo W.H. Molecular cloning and characterization of the gene encoding a fibrinolytic enzyme from Bacillus subtilis strain A1. World J. Microbiol. Biotechnol. 2004;20:711–717. doi: 10.1007/s11274-003-4514-5. - DOI
-
- Mukherjee A.K., Rai S.K., Thakur R., Chattopadhyay P., Kar S.K. Bafibrinase: A non-toxic, non-hemorrhagic, direct-acting fibrinolytic serine protease from Bacillus sp. strain AS-S20-I exhibits in vivo anticoagulant activity and thrombolytic potency. Biochimie. 2012;94:1300–1308. doi: 10.1016/j.biochi.2012.02.027. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

