Metabolic Alterations in Pancreatic Cancer Progression
- PMID: 31861288
- PMCID: PMC7016676
- DOI: 10.3390/cancers12010002
Metabolic Alterations in Pancreatic Cancer Progression
Abstract
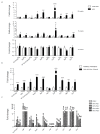
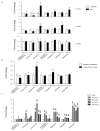
Pancreatic cancer is the third leading cause of cancer-related deaths in the USA. Pancreatic tumors are characterized by enhanced glycolytic metabolism promoted by a hypoxic tumor microenvironment and a resultant acidic milieu. The metabolic reprogramming allows cancer cells to survive hostile microenvironments. Through the analysis of the principal metabolic pathways, we identified the specific metabolites that are altered during pancreatic cancer progression in the spontaneous progression (KPC) mouse model. Genetically engineered mice exhibited metabolic alterations during PanINs formation, even before the tumor development. To account for other cells in the tumor microenvironment and to focus on metabolic adaptations concerning tumorigenic cells only, we compared the metabolic profile of KPC and orthotopic tumors with those obtained from KPC-tumor derived cell lines. We observed significant upregulation of glycolysis and the pentose phosphate pathway metabolites even at the early stages of pathogenesis. Other biosynthetic pathways also demonstrated a few common perturbations. While some of the metabolic changes in tumor cells are not detectable in orthotopic and spontaneous tumors, a significant number of tumor cell-intrinsic metabolic alterations are readily detectable in the animal models. Overall, we identified that metabolic alterations in precancerous lesions are maintained during cancer development and are largely mirrored by cancer cells in culture conditions.
Keywords: KPC mice; cancer metabolism; metabolic alterations; pancreatic cancer; precancerous lesions.
Conflict of interest statement
The authors declare no conflict of interests.
Figures




References
-
- Lucas A.L., Shakya R., Lipsyc M.D., Mitchel E.B., Kumar S., Hwang C., Deng L., Devoe C., Chabot J.A., Szabolcs M., et al. High prevalence of BRCA1 and BRCA2 germline mutations with loss of heterozygosity in a series of resected pancreatic adenocarcinoma and other neoplastic lesions. Clin. Cancer Res. 2013;19:3396–3403. doi: 10.1158/1078-0432.CCR-12-3020. - DOI - PMC - PubMed
-
- Di Marco M., Astolfi A., Grassi E., Vecchiarelli S., Macchini M., Indio V., Casadei R., Ricci C., D’Ambra M., Taffurelli G., et al. Characterization of pancreatic ductal adenocarcinoma using whole transcriptome sequencing and copy number analysis by single-nucleotide polymorphism array. Mol. Med. Rep. 2015;12:7479–7484. doi: 10.3892/mmr.2015.4344. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

