Peroxiredoxin II Maintains the Mitochondrial Membrane Potential against Alcohol-Induced Apoptosis in HT22 Cells
- PMID: 31861323
- PMCID: PMC7023630
- DOI: 10.3390/antiox9010001
Peroxiredoxin II Maintains the Mitochondrial Membrane Potential against Alcohol-Induced Apoptosis in HT22 Cells
Abstract
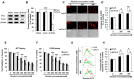
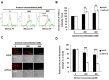
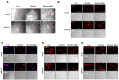
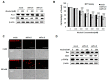
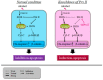
Excessive alcohol intake can significantly reduce cognitive function and cause irreversible learning and memory disorders. The brain is particularly vulnerable to alcohol-induced ROS damage; the hippocampus is one of the most sensitive areas of the brain for alcohol neurotoxicity. In the present study, we observed significant increasing of intracellular ROS accumulations in Peroxiredoxin II (Prx II) knockdown HT22 cells, which were induced by alcohol treatments. We also found that the level of ROS in mitochondrial was also increased, resulting in a decrease in the mitochondrial membrane potential. The phosphorylation of GSK3β (Ser9) and anti-apoptotic protein Bcl2 expression levels were significantly downregulated in Prx II knockdown HT22 cells, which suggests that Prx II knockdown HT22 cells were more susceptible to alcohol-induced apoptosis. Scavenging the alcohol-induced ROS with NAC significantly decreased the intracellular ROS levels, as well as the phosphorylation level of GSK3β in Prx II knockdown HT22 cells. Moreover, NAC treatment also dramatically restored the mitochondrial membrane potential and the cellular apoptosis in Prx II knockdown HT22 cells. Our findings suggest that Prx II plays a crucial role in alcohol-induced neuronal cell apoptosis by regulating the cellular ROS levels, especially through regulating the ROS-dependent mitochondrial membrane potential. Consequently, Prx II may be a therapeutic target molecule for alcohol-induced neuronal cell death, which is closely related to ROS-dependent mitochondria dysfunction.
Keywords: Prx II; ROS; alcohol; neuronal cell; oxidative damage.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


References
-
- Gonzalez-Reimers E., Camino M., Fernández-Rodríguez M., Martín-González C., Hernández-Betancor I., Abreu-González P., de la Vega-Prieto M.J., Elvira-Cabrera O., Santolaria-Fernández F. Antioxidant vitamins and brain dysfunction in alcoholics. Alcohol Alcohol. 2014;49:45–50. doi: 10.1093/alcalc/agt150. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

