Tetrahydroquinoline-Isoxazole/Isoxazoline Hybrid Compounds as Potential Cholinesterases Inhibitors: Synthesis, Enzyme Inhibition Assays, and Molecular Modeling Studies
- PMID: 31861333
- PMCID: PMC6981637
- DOI: 10.3390/ijms21010005
Tetrahydroquinoline-Isoxazole/Isoxazoline Hybrid Compounds as Potential Cholinesterases Inhibitors: Synthesis, Enzyme Inhibition Assays, and Molecular Modeling Studies
Abstract
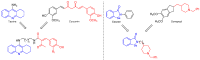
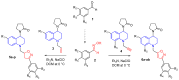
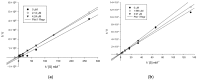
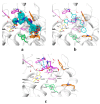
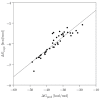
A series of 44 hybrid compounds that included in their structure tetrahydroquinoline (THQ) and isoxazole/isoxazoline moieties were synthesized through the 1,3-dipolar cycloaddition reaction (1,3-DC) from the corresponding N-allyl/propargyl THQs, previously obtained via cationic Povarov reaction. In vitro cholinergic enzymes inhibition potential of all compounds was tested. Enzyme inhibition assays showed that some hybrids exhibited significant potency to inhibit acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). Especially, the hybrid compound 5n presented the more effective inhibition against AChE (4.24 µM) with an acceptable selectivity index versus BChE (SI: 5.19), while compound 6aa exhibited the greatest inhibition activity on BChE (3.97 µM) and a significant selectivity index against AChE (SI: 0.04). Kinetic studies were carried out for compounds with greater inhibitory activity of cholinesterases. Structure-activity relationships of the molecular hybrids were analyzed, through computational models using a molecular cross-docking algorithm and Molecular Mechanics/Generalized Born Surface Area (MM/GBSA) binding free energy approach, which indicated a good correlation between the experimental inhibition values and the predicted free binding energy.
Keywords: Alzheimer’s disease; cholinesterase inhibitors; cross-docking and MM/GBSA free binding energy; hybrid compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Di Pietro O., Viayna E., Vicente-Garcia E., Bartolini M., Ramon R., Juarez-Jimenez J., Clos M.V., Perez B., Andrisano V., Luque F.J., et al. 1,2,3,4-Tetrahydrobenzo [H] [1,6] naphthyridines as a new family of potent peripheral-to-midgorge-site inhibitors of acetylcholinesterase: Synthesis, pharmacological evaluation and mechanistic studies. Eur. J. Med. Chem. 2014;73:141–152. doi: 10.1016/j.ejmech.2013.12.008. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

