A Critical Review on the Production of Electrospun Nanofibres for Guided Bone Regeneration in Oral Surgery
- PMID: 31861582
- PMCID: PMC7023267
- DOI: 10.3390/nano10010016
A Critical Review on the Production of Electrospun Nanofibres for Guided Bone Regeneration in Oral Surgery
Abstract
Nanofibre-based membranes or scaffolds exhibit high surface-to-volume ratio, which allows an improved cell adhesion, representing an attractive subgroup of biomaterials due to their unique properties. Among several techniques of nanofiber production, electrospinning is a cost-effective technique that has been, to date, attractive for several medical applications. Among these, guided bone regeneration is a surgical procedure in which bone regeneration, due to bone atrophy following tooth loss, is "guided" by an occlusive barrier. The membrane should protect the initial blood clot from any compression, shielding the bone matrix during maturation from infiltration of soft tissues cells. This review will focus its attention on the application of electrospinning (ELS) in oral surgery bone regeneration. Despite the abundance of published papers related to the electrospinning technique applied in the field of bone regeneration of the jaws, to the authors' knowledge, no articles report clinical application of these structures. Moreover, only a few records can be found with in vivo application. Therefore, no human studies have to date been detectable. New approaches such as multifunctional multilayering and coupling with bone promoting factors or antimicrobial agents, makes this technology very attractive. However, greater efforts should be made by researchers and companies to turn these results into clinical practice.
Keywords: electrospinning; guided bone regeneration; membranes; oral surgery; scaffolds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
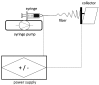





References
-
- Ma P.X. Scaffolds for tissue fabrication. Mater. Today. 2004;7:30–40. doi: 10.1016/S1369-7021(04)00233-0. - DOI
-
- Zhang L., Webster T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today. 2009;4:66–80. doi: 10.1016/j.nantod.2008.10.014. - DOI
-
- Rahman S.U., Oh J.H., Cho Y.D., Chung S.H., Lee G., Baek J.H., Ryoo H.M., Woo K.M. Fibrous Topography-Potentiated Canonical Wnt Signaling Directs the Odontoblastic Differentiation of Dental Pulp-Derived Stem Cells. ACS Appl. Mater. Interfaces. 2018;10:17526–17541. doi: 10.1021/acsami.7b19782. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

