Synthesis and Application of 1,2-Aminoalcohols with Neoisopulegol-Based Octahydrobenzofuran Core
- PMID: 31861609
- PMCID: PMC6982906
- DOI: 10.3390/molecules25010021
Synthesis and Application of 1,2-Aminoalcohols with Neoisopulegol-Based Octahydrobenzofuran Core
Abstract
A library of 1,2-aminoalcohol derivatives with a neoisopulegol-based octahydrobenzofuran core was developed and applied as chiral catalysts in the addition of diethylzinc to benzaldehyde. The allylic chlorination of (+)-neoisopulegol, derived from natural (-)-isopulegol followed by cyclization, gave the key methyleneoctahydrobenzofuran intermediate. The stereoselective epoxidation of the key intermediate and subsequent oxirane ring opening with primary amines afforded the required 1,2-aminoalcohols. The ring closure of the secondary amine analogues with formaldehyde provided spiro-oxazolidine ring systems. The dihydroxylation of the methylenetetrahydrofuran moiety with OsO4/NMO (4-methylmorpholine N-oxide) resulted in the formation of a neoisopulegol-based diol in a highly stereoselective reaction. The antimicrobial activity of both the aminoalcohol derivatives and the diol was also explored.
Keywords: 1,2-aminoalcohol; antimicrobial activity; chiral catalyst; neoisopulegol; octahydrobenzofuran.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
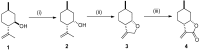

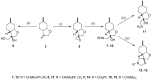


References
-
- Keay B.A., Hopkins J.M., Dibble P.W. Furans and their benzo derivatives: Applications. In: Katritzky A.R., Ramsden C.A., Scriven E.F.V., Taylor R.J.K., editors. Comprehensive Heterocyclic Chemistry III. 1st ed. Volume 5. Elsevier; Amsterdam, The Netherlands: 2008. pp. 571–623.
-
- Keay B.A., Dibble P.W. Furans and their benzo derivatives: Applications. In: Katritzky A.R., Rees C.V., Scriven E.F.V., editors. Comprehensive Heterocyclic Chemistry II. 2nd ed. Volume 2. Elsevier; Amsterdam, The Netherlands: 1996. pp. 395–436.
-
- Zhang Y., Negishi E. Metal-promoted cyclization. 25. Palladium-catalyzed cascade carbometalation of alkynes and alkenes as an efficient route to cyclic and polycyclic structures. J. Am. Chem. Soc. 1989;111:3454–3456. doi: 10.1021/ja00191a066. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

