Cellular microRNA-155 Regulates Virus-Induced Inflammatory Response and Protects against Lethal West Nile Virus Infection
- PMID: 31861621
- PMCID: PMC7019255
- DOI: 10.3390/v12010009
Cellular microRNA-155 Regulates Virus-Induced Inflammatory Response and Protects against Lethal West Nile Virus Infection
Abstract
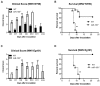
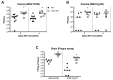
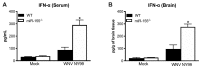
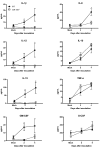
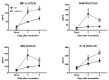
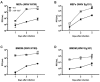
West Nile virus (WNV) is a flavivirus that has disseminated globally as a significant cause of viral encephalitis in humans. MircoRNA-155 (miR-155) regulates various aspects of innate and adaptive immune responses. We previously reported that WNV infection induces upregulation of miR-155 in mice brains. In the current study, we demonstrate the critical role of miR-155 in restricting the pathogenesis of WNV infection in mice. Compared to wild-type (WT) mice, miR-155 knockout mice exhibited significantly higher morbidity and mortality after infection with either a lethal strain, WNV NY99, or a non-lethal strain, WNV Eg101. Increased mortality in miR-155-/- mice was associated with significantly high WNV burden in the serum and brains. Protein levels of interferon (IFN)-α in the serum and brains were higher in miR-155-/- mice. However, miR-155-/- mice exhibited significantly lower protein levels of anti-viral interleukin (IL)-1β, IL-12, IL-6, IL-15, and GM-CSF despite the high viral load. Primary mouse cells lacking miR-155 were more susceptible to infection with WNV compared to cells derived from WT mice. Besides, overexpression of miR-155 in human neuronal cells modulated anti-viral cytokine response and resulted in significantly lower WNV replication. These data collectively indicate that miR-155 restricts WNV production in mouse and human cells and protects against lethal WNV infection in mice.
Keywords: West Nile virus; immune response; inflammatory cytokines and chemokines; miR-155; micro-RNAs; virus replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Induction of virus-specific effector immune cell response limits virus replication and severe disease in mice infected with non-lethal West Nile virus Eg101 strain.J Neuroinflammation. 2015 Sep 22;12:178. doi: 10.1186/s12974-015-0400-y. J Neuroinflammation. 2015. PMID: 26392176 Free PMC article.
-
The critical role of interleukin-6 in protection against neurotropic flavivirus infection.Front Cell Infect Microbiol. 2023 Nov 16;13:1275823. doi: 10.3389/fcimb.2023.1275823. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38053527 Free PMC article.
-
Infection with non-lethal West Nile virus Eg101 strain induces immunity that protects mice against the lethal West Nile virus NY99 strain.Viruses. 2014 Jun 6;6(6):2328-39. doi: 10.3390/v6062328. Viruses. 2014. PMID: 24915459 Free PMC article.
-
Role of Immune Aging in Susceptibility to West Nile Virus.Methods Mol Biol. 2016;1435:235-47. doi: 10.1007/978-1-4939-3670-0_18. Methods Mol Biol. 2016. PMID: 27188562 Free PMC article. Review.
-
The innate immune playbook for restricting West Nile virus infection.Viruses. 2013 Oct 30;5(11):2643-58. doi: 10.3390/v5112643. Viruses. 2013. PMID: 24178712 Free PMC article. Review.
Cited by
-
The miRNA: a small but powerful RNA for COVID-19.Brief Bioinform. 2021 Mar 22;22(2):1137-1149. doi: 10.1093/bib/bbab062. Brief Bioinform. 2021. PMID: 33675361 Free PMC article. Review.
-
Involvement of host microRNAs in flavivirus-induced neuropathology: An update.J Biosci. 2022;47(3):54. doi: 10.1007/s12038-022-00288-1. J Biosci. 2022. PMID: 36222134 Free PMC article. Review.
-
Host Combats IBDV Infection at Both Protein and RNA Levels.Viruses. 2022 Oct 21;14(10):2309. doi: 10.3390/v14102309. Viruses. 2022. PMID: 36298864 Free PMC article. Review.
-
The Role of Noncoding RNA in the Transmission and Pathogenicity of Flaviviruses.Viruses. 2024 Feb 2;16(2):242. doi: 10.3390/v16020242. Viruses. 2024. PMID: 38400018 Free PMC article. Review.
-
Micro-Players of Great Significance-Host microRNA Signature in Viral Infections in Humans and Animals.Int J Mol Sci. 2022 Sep 11;23(18):10536. doi: 10.3390/ijms231810536. Int J Mol Sci. 2022. PMID: 36142450 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

