Muraymycin Nucleoside Antibiotics: Structure-Activity Relationship for Variations in the Nucleoside Unit
- PMID: 31861655
- PMCID: PMC6983020
- DOI: 10.3390/molecules25010022
Muraymycin Nucleoside Antibiotics: Structure-Activity Relationship for Variations in the Nucleoside Unit
Abstract
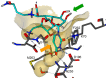
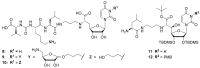
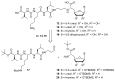
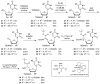
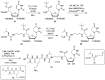
Muraymycins are a subclass of naturally occurring nucleoside antibiotics with promising antibacterial activity. They inhibit the bacterial enzyme translocase I (MraY), a clinically yet unexploited target mediating an essential intracellular step of bacterial peptidoglycan biosynthesis. Several structurally simplified muraymycin analogues have already been synthesized for structure-activity relationship (SAR) studies. We now report on novel derivatives with unprecedented variations in the nucleoside unit. For the synthesis of these new muraymycin analogues, we employed a bipartite approach facilitating the introduction of different nucleosyl amino acid motifs. This also included thymidine- and 5-fluorouridine-derived nucleoside core structures. Using an in vitro assay for MraY activity, it was found that the introduction of substituents in the 5-position of the pyrimidine nucleobase led to a significant loss of inhibitory activity towards MraY. The loss of nucleobase aromaticity (by reduction of the uracil C5-C6 double bond) resulted in a ca. tenfold decrease in inhibitory potency. In contrast, removal of the 2'-hydroxy group furnished retained activity, thus demonstrating that modifications of the ribose moiety might be well-tolerated. Overall, these new SAR insights will guide the future design of novel muraymycin analogues for their potential development towards antibacterial drug candidates.
Keywords: antibiotics; natural products; nucleoside analogues; structure–activity relationships.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


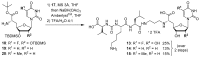
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

