The Molecular Mechanisms Underlying Iron Deficiency Responses in Rice
- PMID: 31861687
- PMCID: PMC6981701
- DOI: 10.3390/ijms21010043
The Molecular Mechanisms Underlying Iron Deficiency Responses in Rice
Abstract
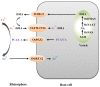
Iron (Fe) is an essential element required for plant growth and development. Under Fe-deficientconditions, plants have developed two distinct strategies (designated as strategy I and II) to acquire Fe from soil. As a graminaceous species, rice is not a typical strategy II plant, as it not only synthesizes DMA (2'-deoxymugineic acid) in roots to chelate Fe3+ but also acquires Fe2+ through transporters OsIRT1 and OsIRT2. During the synthesis of DMA in rice, there are three sequential enzymatic reactions catalyzed by enzymes NAS (nicotianamine synthase), NAAT (nicotianamine aminotransferase), and DMAS (deoxymugineic acid synthase). Many transporters required for Fe uptake from the rhizosphere and internal translocation have also been identified in rice. In addition, the signaling networks composed of various transcription factors (such as IDEF1, IDEF2, and members of the bHLH (basic helix-loop-helix) family), phytohormones, and signaling molecules are demonstrated to regulate Fe uptake and translocation. This knowledge greatly contributes to our understanding of the molecular mechanisms underlying iron deficiency responses in rice.
Keywords: Fe acquisition; phytohormones; rice (Oryza sativa), Fe deficiency; strategy II; transcription factors; transporters.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Paralogs and mutants show that one DMA synthase functions in iron homeostasis in rice.J Exp Bot. 2017 Mar 1;68(7):1785-1795. doi: 10.1093/jxb/erx065. J Exp Bot. 2017. PMID: 28369596 Free PMC article.
-
Deoxymugineic acid increases Zn translocation in Zn-deficient rice plants.Plant Mol Biol. 2008 Apr;66(6):609-17. doi: 10.1007/s11103-008-9292-x. Epub 2008 Jan 26. Plant Mol Biol. 2008. PMID: 18224446 Free PMC article.
-
Mutation in nicotianamine aminotransferase stimulated the Fe(II) acquisition system and led to iron accumulation in rice.Plant Physiol. 2007 Dec;145(4):1647-57. doi: 10.1104/pp.107.107912. Epub 2007 Oct 19. Plant Physiol. 2007. PMID: 17951455 Free PMC article.
-
Route and Regulation of Zinc, Cadmium, and Iron Transport in Rice Plants (Oryza sativa L.) during Vegetative Growth and Grain Filling: Metal Transporters, Metal Speciation, Grain Cd Reduction and Zn and Fe Biofortification.Int J Mol Sci. 2015 Aug 13;16(8):19111-29. doi: 10.3390/ijms160819111. Int J Mol Sci. 2015. PMID: 26287170 Free PMC article. Review.
-
The Adaptive Mechanism of Plants to Iron Deficiency via Iron Uptake, Transport, and Homeostasis.Int J Mol Sci. 2019 May 16;20(10):2424. doi: 10.3390/ijms20102424. Int J Mol Sci. 2019. PMID: 31100819 Free PMC article. Review.
Cited by
-
Mapping of the Quantitative Trait Loci and Candidate Genes Associated With Iron Efficiency in Maize.Front Plant Sci. 2022 Apr 22;13:855572. doi: 10.3389/fpls.2022.855572. eCollection 2022. Front Plant Sci. 2022. PMID: 35528939 Free PMC article.
-
Genome-Wide Identification of Wheat ZIP Gene Family and Functional Characterization of the TaZIP13-B in Plants.Front Plant Sci. 2021 Nov 3;12:748146. doi: 10.3389/fpls.2021.748146. eCollection 2021. Front Plant Sci. 2021. PMID: 34804090 Free PMC article.
-
A Glucuronic Acid-Producing Endophyte Pseudomonas sp. MCS15 Reduces Cadmium Uptake in Rice by Inhibition of Ethylene Biosynthesis.Front Plant Sci. 2022 Apr 14;13:876545. doi: 10.3389/fpls.2022.876545. eCollection 2022. Front Plant Sci. 2022. PMID: 35498658 Free PMC article.
-
Genome-Wide Association Analysis Reveals the Genetic Basis of Iron-Deficiency Stress Tolerance in Maize.Front Plant Sci. 2022 Jun 2;13:878809. doi: 10.3389/fpls.2022.878809. eCollection 2022. Front Plant Sci. 2022. PMID: 35720580 Free PMC article.
-
An integration of genome-wide survey, homologous comparison and gene expression analysis provides a basic framework for the ZRT, IRT-like protein (ZIP) in foxtail millet.Front Plant Sci. 2024 Sep 5;15:1467015. doi: 10.3389/fpls.2024.1467015. eCollection 2024. Front Plant Sci. 2024. PMID: 39301166 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

