Myeloid Differentiation Protein 2 Mediates Angiotensin II-Induced Liver Inflammation and Fibrosis in Mice
- PMID: 31861702
- PMCID: PMC6983196
- DOI: 10.3390/molecules25010025
Myeloid Differentiation Protein 2 Mediates Angiotensin II-Induced Liver Inflammation and Fibrosis in Mice
Abstract
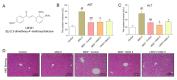
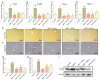
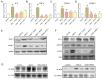
Angiotensin II (Ang II) participates in the pathogenesis of liver injury. Our previous publications reported that myeloid differentiation protein 2 (MD2) mediates Ang II-induced cardiac and kidney inflammation by directly binding to Ang II. Thus, we hypothesize that MD2 is critical to Ang II-induced liver injury. Subcutaneous injections of Ang II for 8 weeks were adopted to build the liver injury model. With a specific MD2 inhibitor L6H21 and MD2 knockout mice, we reported that MD2 inhibition and knockout significantly mitigate liver inflammation and fibrosis in mice injected with Ang II. To be more specific, the functional and pathological damages induced by Ang II were mitigated by L6H21 or MD2 knockout. MD2 knockout or L6H21 administration inhibited the Ang II-induced upregulation of fibrosis markers, inflammatory cytokines, and adhesion molecules in gene or protein levels. The activation of NF-κB and Extracellular signal-regulated kinases (ERK) induced by Ang II was also reversed by L6H21 treatment or MD2 deficiency. Note that the co-immunoprecipitation study showed that L6H21 downregulated the ANG II-induced toll-like receptor 4 (TLR4)/MD2 complex in liver tissues while having no effects on MD2 expression. Our results reported the critical role of MD2 in the progress of liver injury and suggested that MD2 is a potential therapeutic target for liver injury.
Keywords: Angiotensin II; L6H21; inflammation; liver injury; myeloid differentiation 2.
Conflict of interest statement
The inventors of L6H21 agree with the publication of this article.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

