Challenges Faced with Small Molecular Modulators of Potassium Current Channel Isoform Kv1.5
- PMID: 31861703
- PMCID: PMC7022446
- DOI: 10.3390/biom10010010
Challenges Faced with Small Molecular Modulators of Potassium Current Channel Isoform Kv1.5
Abstract
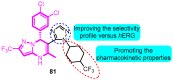
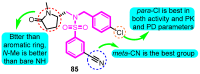
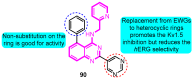
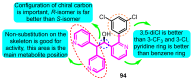
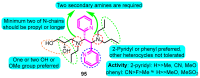
The voltage-gated potassium channel Kv1.5, which mediates the cardiac ultra-rapid delayed-rectifier (IKur) current in human cells, has a crucial role in atrial fibrillation. Therefore, the design of selective Kv1.5 modulators is essential for the treatment of pathophysiological conditions involving Kv1.5 activity. This review summarizes the progress of molecular structures and the functionality of different types of Kv1.5 modulators, with a focus on clinical cardiovascular drugs and a number of active natural products, through a summarization of 96 compounds currently widely used. Furthermore, we also discuss the contributions of Kv1.5 and the regulation of the structure-activity relationship (SAR) of synthetic Kv1.5 inhibitors in human pathophysiology. SAR analysis is regarded as a useful strategy in structural elucidation, as it relates to the characteristics that improve compounds targeting Kv1.5. Herein, we present previous studies regarding the structural, pharmacological, and SAR information of the Kv1.5 modulator, through which we can assist in identifying and designing potent and specific Kv1.5 inhibitors in the treatment of diseases involving Kv1.5 activity.
Keywords: KCNA5; Kv1.5; SAR; modulators; potassium channel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



















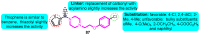




Similar articles
-
Role for myosin-V motor proteins in the selective delivery of Kv channel isoforms to the membrane surface of cardiac myocytes.Circ Res. 2014 Mar 14;114(6):982-92. doi: 10.1161/CIRCRESAHA.114.302711. Epub 2014 Feb 7. Circ Res. 2014. PMID: 24508725 Free PMC article.
-
Pharmacology of voltage-gated potassium channel Kv1.5--impact on cardiac excitability.Curr Opin Pharmacol. 2014 Apr;15:115-21. doi: 10.1016/j.coph.2014.02.001. Epub 2014 Mar 13. Curr Opin Pharmacol. 2014. PMID: 24632326 Review.
-
Inhibitory effects of hesperetin on Kv1.5 potassium channels stably expressed in HEK 293 cells and ultra-rapid delayed rectifier K(+) current in human atrial myocytes.Eur J Pharmacol. 2016 Oct 15;789:98-108. doi: 10.1016/j.ejphar.2016.07.015. Epub 2016 Jul 7. Eur J Pharmacol. 2016. PMID: 27397430
-
Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation.Hum Mol Genet. 2006 Jul 15;15(14):2185-91. doi: 10.1093/hmg/ddl143. Epub 2006 Jun 13. Hum Mol Genet. 2006. PMID: 16772329
-
New drugs targeting the cardiac ultra-rapid delayed-rectifier current (I Kur): rationale, pharmacology and evidence for potential therapeutic value.J Cardiovasc Pharmacol. 2008 Aug;52(2):105-20. doi: 10.1097/FJC.0b013e3181719b0c. J Cardiovasc Pharmacol. 2008. PMID: 18670369 Review.
Cited by
-
Rational Design and Synthesis of 3-Morpholine Linked Aromatic-Imino-1H-Indoles as Novel Kv1.5 Channel Inhibitors Sharing Vasodilation Effects.Front Mol Biosci. 2022 Jan 24;8:805594. doi: 10.3389/fmolb.2021.805594. eCollection 2021. Front Mol Biosci. 2022. PMID: 35141279 Free PMC article.
-
Methods of prolonging the effect of caudal block in children.Front Pediatr. 2024 Jun 3;12:1406263. doi: 10.3389/fped.2024.1406263. eCollection 2024. Front Pediatr. 2024. PMID: 38887564 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

