Glycomic Signatures of Plasma IgG Improve Preoperative Prediction of the Invasiveness of Small Lung Nodules
- PMID: 31861777
- PMCID: PMC6982969
- DOI: 10.3390/molecules25010028
Glycomic Signatures of Plasma IgG Improve Preoperative Prediction of the Invasiveness of Small Lung Nodules
Abstract
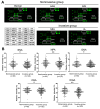
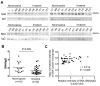
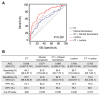
Preoperative assessment of tumor invasiveness is essential to avoid overtreatment for patients with small-sized ground-glass nodules (GGNs) of 10 mm or less in diameter. However, it is difficult to determine the pathological state by computed tomography (CT) examination alone. Aberrant glycans has emerged as a tool to identify novel potential disease biomarkers. In this study, we used a lectin microarray-based strategy to investigate whether glycosylation changes in plasma immunoglobulin G (IgG) provide additional information about the invasiveness of small GGNs before surgery. Two independent cohorts (discovery set, n = 92; test set, n = 210) of GGN patients were used. Five of 45 lectins (Sambucus nigra agglutinin, SNA; Datura stramonium agglutinin, DSA; Galanthus nivalis agglutinin, GNA; Euonymus europaeus lectin, EEL; and Vicia villosa agglutinin, VVA) were identified as independent factors associated with pathological invasiveness of small GGNs (p < 0.01). Receiver-operating characteristic (ROC) curve analysis indicated the combination of these five lectins could significantly improve the accuracy of CT in diagnosing invasive GGNs, with an area under the curve (AUC) of 0.792 (p < 0.001), a sensitivity of 74.6%, and specificity of 74.4%, which was superior to current clinical biomarkers. These results suggest that the multilectin assay based on plasma IgG glycosylation may be a useful in vitro complementary test to enhance preoperative determination of the invasiveness of GGNs and guide surgeons to select proper clinical management to avoid overtreatment.
Keywords: glycobiomarker; ground glass nodule (GGN); immunoglobulin G (IgG); lectin microarray; multilectin assay.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Aberrant glycosylation of the anti-Thomsen-Friedenreich glycotope immunoglobulin G in gastric cancer patients.World J Gastroenterol. 2013 Jun 21;19(23):3573-82. doi: 10.3748/wjg.v19.i23.3573. World J Gastroenterol. 2013. PMID: 23801858 Free PMC article.
-
Radiomics of Vascular Structures in Pulmonary Ground-Glass Nodules: A Predictor of Invasiveness : Radiomics of Vascular Structures in GGNs for Tumor Invasiveness Prediction.Curr Med Imaging. 2025 Apr 21. doi: 10.2174/0115734056385352250410053810. Online ahead of print. Curr Med Imaging. 2025. PMID: 40264304
-
Changes in quantitative CT image features of ground-glass nodules in differentiating invasive pulmonary adenocarcinoma from benign and in situ lesions: histopathological comparisons.Clin Radiol. 2018 May;73(5):504.e9-504.e16. doi: 10.1016/j.crad.2017.12.011. Epub 2018 Jan 9. Clin Radiol. 2018. PMID: 29329732
-
Lung Adenocarcinoma Manifesting as Ground-Glass Opacity Nodules 3 cm or Smaller: Evaluation With Combined High-Resolution CT and PET/CT Modality.AJR Am J Roentgenol. 2019 Nov;213(5):W236-W245. doi: 10.2214/AJR.19.21382. Epub 2019 Jul 30. AJR Am J Roentgenol. 2019. PMID: 31361533
-
Identification of preoperative prediction factors of tumor subtypes for patients with solitary ground-glass opacity pulmonary nodules.J Cardiothorac Surg. 2018 Jan 17;13(1):9. doi: 10.1186/s13019-018-0696-7. J Cardiothorac Surg. 2018. PMID: 29343293 Free PMC article.
Cited by
-
Differential Glycoform Analysis of MUC1 Derived from Biological Specimens Using an Antibody-Overlay Lectin Microarray.Methods Mol Biol. 2024;2763:223-236. doi: 10.1007/978-1-0716-3670-1_19. Methods Mol Biol. 2024. PMID: 38347414
-
Differential Glycosylation Levels in Saliva from Patients with Lung or Breast Cancer: A Preliminary Assessment for Early Diagnostic Purposes.Metabolites. 2021 Aug 24;11(9):566. doi: 10.3390/metabo11090566. Metabolites. 2021. PMID: 34564382 Free PMC article.
-
Bakuchicin alleviates ovalbumin-induced allergic asthma by regulating M2 macrophage polarization.Inflamm Res. 2024 May;73(5):725-737. doi: 10.1007/s00011-024-01859-8. Epub 2024 Mar 27. Inflamm Res. 2024. PMID: 38538755
-
Pharmacotherapeutic Strategies for Fine Particulate Matter-Induced Lung and Cardiovascular Damage: Marketed Drugs, Traditional Chinese Medicine, and Biological Agents.Cardiovasc Toxicol. 2025 May;25(5):666-691. doi: 10.1007/s12012-025-09985-3. Epub 2025 Mar 20. Cardiovasc Toxicol. 2025. PMID: 40113640 Review.
-
Databases and Bioinformatic Tools for Glycobiology and Glycoproteomics.Int J Mol Sci. 2020 Sep 14;21(18):6727. doi: 10.3390/ijms21186727. Int J Mol Sci. 2020. PMID: 32937895 Free PMC article. Review.
References
-
- Travis W.D., Brambilla E., Noguchi M., Nicholson A.G., Geisinger K.R., Yatabe Y., Beer D.G., Powell C.A., Riely G.J., Van Schil P.E., et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J. Thorac. Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2018ZX10302205/National Science and Technology Major Project of China
- 2012AA020203/National High Technology Research and Development Program of China
- 31570796, 31770850 and 81802100/National Natural Science Foundation of China
- 18YF1410500/Shanghai Sailing Program
- YG2015ZD14, YG2017MS63 and YG2016QN58/Shanghai Jiao Tong University Interdiscipline with Medicine Program
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

