DRG2 Deficient Mice Exhibit Impaired Motor Behaviors with Reduced Striatal Dopamine Release
- PMID: 31861806
- PMCID: PMC6981536
- DOI: 10.3390/ijms21010060
DRG2 Deficient Mice Exhibit Impaired Motor Behaviors with Reduced Striatal Dopamine Release
Abstract
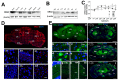
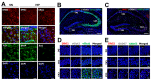
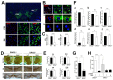
Developmentally regulated GTP-binding protein 2 (DRG2) was first identified in the central nervous system of mice. However, the physiological function of DRG2 in the brain remains largely unknown. Here, we demonstrated that knocking out DRG2 impairs the function of dopamine neurons in mice. DRG2 was strongly expressed in the neurons of the dopaminergic system such as those in the striatum (Str), ventral tegmental area (VTA), and substantia nigra (SN), and on neuronal cell bodies in high-density regions such as the hippocampus (HIP), cerebellum, and cerebral cortex in the mouse brain. DRG2 knockout (KO) mice displayed defects in motor function in motor coordination and rotarod tests and increased anxiety. However, unexpectedly, DRG2 depletion did not affect the dopamine (DA) neuron population in the SN, Str, or VTA region or dopamine synthesis in the Str region. We further demonstrated that dopamine release was significantly diminished in the Str region of DRG2 KO mice and that treatment of DRG2 KO mice with l-3,4-dihydroxyphenylalanine (L-DOPA), a dopamine precursor, rescued the behavioral motor deficiency in DRG2 KO mice as observed with the rotarod test. This is the first report to identify DRG2 as a key regulator of dopamine release from dopamine neurons in the mouse brain.
Keywords: Developmentally regulated GTP-binding protein 2 (DRG2); Dopamine release; Dopaminergic neurons; Motor coordination; Motor deficiency; Striatum.
Conflict of interest statement
These authors declare no conflicts of interest.
Figures


Similar articles
-
Pitx3 deficiency produces decreased dopamine signaling and induces motor deficits in Pitx3(-/-) mice.Neurobiol Aging. 2015 Dec;36(12):3314-3320. doi: 10.1016/j.neurobiolaging.2015.08.012. Epub 2015 Aug 20. Neurobiol Aging. 2015. PMID: 26363812 Free PMC article.
-
Neuronal KIF5b deletion induces striatum-dependent locomotor impairments and defects in membrane presentation of dopamine D2 receptors.J Neurochem. 2019 May;149(3):362-380. doi: 10.1111/jnc.14665. Epub 2019 Feb 14. J Neurochem. 2019. PMID: 30664247
-
Segregation of dopamine and glutamate release sites in dopamine neuron axons: regulation by striatal target cells.FASEB J. 2019 Jan;33(1):400-417. doi: 10.1096/fj.201800713RR. Epub 2018 Jul 16. FASEB J. 2019. PMID: 30011230
-
Conditional deletion of Ndufs4 in dopaminergic neurons promotes Parkinson's disease-like non-motor symptoms without loss of dopamine neurons.Sci Rep. 2017 Mar 22;7:44989. doi: 10.1038/srep44989. Sci Rep. 2017. PMID: 28327638 Free PMC article.
-
α2A adrenergic receptors highly expressed in mesoprefrontal dopamine neurons.Neuroscience. 2016 Sep 22;332:130-9. doi: 10.1016/j.neuroscience.2016.06.037. Epub 2016 Jun 27. Neuroscience. 2016. PMID: 27365174
Cited by
-
The Effects of Intravermis Cerebellar Microinjections of Dopaminergic Agents in Motor Learning and Aversive Memory Acquisition in Mice.Front Behav Neurosci. 2021 Feb 25;15:628357. doi: 10.3389/fnbeh.2021.628357. eCollection 2021. Front Behav Neurosci. 2021. PMID: 33716682 Free PMC article.
-
Multiomics Integration of Parkinson's Disease Datasets Reveals Unexpected Roles of IRE1 in Its Pathology.Int J Mol Sci. 2025 Jul 12;26(14):6711. doi: 10.3390/ijms26146711. Int J Mol Sci. 2025. PMID: 40724959 Free PMC article.
-
Inducible Systemic Gcn1 Deletion in Mice Leads to Transient Body Weight Loss upon Tamoxifen Treatment Associated with Decrease of Fat and Liver Glycogen Storage.Int J Mol Sci. 2022 Mar 16;23(6):3201. doi: 10.3390/ijms23063201. Int J Mol Sci. 2022. PMID: 35328622 Free PMC article.
-
Molecular Mechanisms of Neural Circuit Development and Regeneration.Int J Mol Sci. 2021 Apr 27;22(9):4593. doi: 10.3390/ijms22094593. Int J Mol Sci. 2021. PMID: 33925608 Free PMC article.
-
Ribosome binding protein GCN1 regulates the cell cycle and cell proliferation and is essential for the embryonic development of mice.PLoS Genet. 2020 Apr 23;16(4):e1008693. doi: 10.1371/journal.pgen.1008693. eCollection 2020 Apr. PLoS Genet. 2020. PMID: 32324833 Free PMC article.
References
-
- Yang Y., Zhang K., Zhong J., Wang J., Yu Z., Lei X., Chen X., Quan Y., Xian J., Chen Y., et al. Stably maintained microtubules protect dopamine neurons and alleviate depression-like behavior after intracerebral hemorrhage. Sci. Rep. 2018;8:12647. doi: 10.1038/s41598-018-31056-7. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

